Sept. 23 Lecture Notes
advertisement

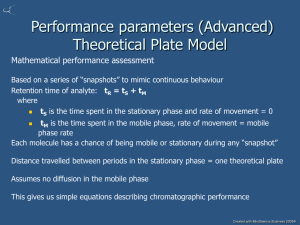
Chem. 230 – 9/23 Lecture Announcements • Exam 1 today – first 40 min. • Second Homework Set will be online soon • Today’s Topics – Chromatographic Theory – Basic definitions (flow – time relationship, distribution constant, retention factor, velocities, plate number, plate height, asymmetry factor, resolution, separation factor) – How to read chromatograms – Meaning of parameters (more when we cover optimization) Chromatographic Theory Questions on Definitions 1. When is chromatographic separation needed vs. only simple separations? 2. An analyte interacts with a stationary phase via adsorption. The stationary phase is most likely: a) Liquid b) Liquid-like c) Solid 3. What are the required two phases in chromatography called? 4. What are advantages and disadvantages with the three common stationary phases (liquid, liquid-like, and solid)? Chromatographic Theory Definition Section – Flow – Volume Relation • Relationship between volume (used with gravity columns) and time (most common with more modern instruments): V = t·F V = volume passing through column part in time t at flow rate F Also, VR = tR·F where R refers to retention time/volume (time it takes component to go through column or volume of solvent needed to elute compound) Chromatographic Theory Definition Section – More on Volume • Hold-up volume = VM = volume occupied by mobile phase in column • Stationary phase volume = VS • Calculation of VM: VM = Vcolumn – Vpacking material – VS VM = tM·F, where tM = time needed for unretained compounds to elute from column Chromatographic Theory Definition Section – Partition and Retention • Distribution Constant (= Partition Coefficient from LLE) = KC = [X]S/[X]M • KC is constant if T and/or solvent remain constant • Retention Factor (= Capacity Factor = Partition Ratio) = k = (moles X)S/(moles X)M = KC/(VM/VS) • k = KC/β where β = VM/VS • Retention Factor is more commonly used because of ease in measuring, and since β = constant, k = constant·KC (for a given column) • Note: kColumn1 ≠ kColumn2 (because β changes) Chromatographic Theory Definition Section – Partition and Retention • Since the fraction of time a solute molecule spends in a given phase is proportional to the fraction of moles in that phase, k = (time in stationary phase)/(time in mobile phase) • Experimentally, k = (tR – tM)/tM • The same equations can be made with volumes instead: k = (VR – VM)/VM • Note: t’R = tR – tM = adjusted retention time Chromatographic Theory Reading Chromatograms • • • • • Determination of parameters from reading chromatogram (HPLC example) tM = 2.37 min. (normally determined by finding 1st peak for unretained compounds – contaminant below) VM = F·tM = (1.0 mL/min)(2.37 min) = 2.37 mL (Note: 4.6 x 250 mm column, so total vol. = (π/4)(0.46 cm)2(25 cm)(1 mL/cm3) = 4.15 mL Vol. of packing material + stationary phase = 4.15 mL – 2.37 mL = 1.78 mL (note only VS is useful) 1st peak, tR = 5.93 min.; k = (5.93 -2.37)/2.37 = 1.50 Chromatographic Theory What do all these Parameters Mean? • KC is just like KP in liquid – liquid extractions for HPLC or KH (Henry’s law constant) for GC • Large KC value means analyte prefers stationary phase • In GC: – KC value will depend on volatility and polarity (analyte vs stationary phase) – KC value adjusted by changing T (most common) – The mobile phase or carrier gas (e.g. He vs. N2) has no effect on KC • In HPLC – KC value will depend on analyte vs. mobile phase and stationary phase polarity – KC value adjusted by changing mobile phase polarity Chromatographic Theory What do all these Parameters Mean? II • Retention Factor is a more useful measure of partitioning because value is related to elution time • Compounds with larger KC, will have larger k, and will elute later • Practical k values – – – – ~0.5 to ~10 Small k values → usually poor selectivity Large k values → must wait long time Higher k values are more practical for complicated samples while low k values are desired for simpler samples to save time Chromatographic Theory Definition Section – Velocity • Mobile phase velocity (u) and analyte average speed (v) can be useful quantities • u = L/tM (L = column length) • v = L/tR • R = retardation factor = v/u (similar to RL used in TLC based on distance migrated) Chromatographic Theory Reading Chromatogram – cont. • u = L/tM = 250 mm/2.37 min = 105 mm/min • v(1st peak) = L/tR = 250 mm/5.93 min = 42.2 mm/min • R = 42.2/105 = 0.40 Chromatographic Theory Shape of Chromatographic Peak • Gaussian Distribution 1 x x 2 1 y exp 2 2 Gaussian Shape (Supposedly) • Normal Distribution Area = 1 • Widths – σ (std deviation) Height – wb (baseline width) = 4σ – wh (peak width at half height) = 2.35σ – w’ = Area/ymax = 2.51σ (often given by integrators) Inflection lines 2σ Half Height wh wb Chromatographic Theory Measures of Chromatographic Efficiency • Plate Number = N (originally number of theoretical plates – similar to number of liquidliquid extractions or distillations) • N = (tR/σ)2 (= 16(tR/wb)2 ) • N is an absolute measure of column efficiency but depends on length • Plate Height = H = length of column needed to get N of 1 • H = L/N, but H is constant under specific conditions, while N is proportional to L Chromatographic Theory Measures of Chromatographic Efficiency • Measuring N and H is valid under isocratic conditions • Later eluting peaks normally used to avoid effects from extra-column broadening • Example: N = 16(14.6/0.9)2 = 4200 (vs. ~3000 for pk 3) • H = L/N = 250 mm/4200 = 0.06 mm Wb ~ 0.9 min Chromatographic Theory Non-ideal Peak Shapes Tailing Peak (actually slow detector) 85 80 Response 75 70 65 60 55 50 45 40 13.00 13.20 13.40 13.60 time a b Tailing Factor = TF = b/a > 1 (tailing peak) 13.80 14.00 Fronting Peak (TF < 1) Chromatographic Theory Definitions - More on Peak Shapes • A Gaussian peak shape is assumed for many of the calculations given previously (e.g. peak width and N) • For non-Gaussian peaks, the equations relating specific widths to σ are no longer valid. • New equations are required for equations that have width in them Chromatographic Theory Definitions - Resolution • Resolution is a measure of the ability to separate two peaks from each other • Resolution = RS d RS wb where d = (tR)B – (tR)A and ave w = [(wb)A + (wb)B]/2 Chromatographic Theory Definitions - Resolution • Resolution indicates the amount of overlap between peaks • RS < 1, means significant overlap • RS = 1.5, means about minimum for “baseline resolution” (at least for two peaks of equal height) • RS > 2 often needed if it is important to integrate a small peak near a large peak Chromatographic Theory Definitions - Resolution • RS calculation examples: – 1st two peaks: • tR(1st pk) = 4.956 min., w (integrator) = w’ = 0.238 min, so wb = 0.238·(4/2.5) = 0.38 min. • tR(2nd pk) = 5.757 min., wb = 0.44 min RS = 0.801/0.410 = 1.95 (neglecting non-Gaussian peak shape) – Last two peaks, RS = 3.0 Chromatographic Theory Definitions - Resolution • Higher resolution values are needed to quantify small peaks next to large peaks • RS = 1.61 (assuming wb 1st peak equals 2nd peak) • RS is not sufficient for accurate integration of 1st peak (but o.k. for integration of 2nd peak) Expansion of above box Large integration error on 1st pk Chromatographic Theory Definitions - Peak Capacity • Peak Capacity is the theoretical maximum number of peaks that can be separated with RS = 1.0 within a given time period. • We won’t cover calculation, but for example, about 2X more peaks could be possible between 5 and 13 min. • Peak capacity 2.3 to 20 min. would be ~27 peaks. • Greater peak capacity is typical with temperature/gradient programs (like in example). Chromatographic Theory Definitions - Separation Factor • Separation Factor = a = ratio of distribution constants a = KB/KA = kB/kA = (t’R)B/(t’R)A Where (tR)B > (tR)A so that a > 1 • Smaller a (closer to 1) means more difficult separation • In example chromatogram, (1st 2 peaks) a = (5.77 – 2.37)/(4.96 – 2.37) = 1.31 Chromatographic Theory Definitions - Overview • The “good” part of chromatography is separation, which results from differences in KC values giving rise to a > 1 • The “bad” part of chromatography is band broadening or dispersion, leading to decreased efficiency (and also reducing sensitivity) • The “ugly” part of chromatography is nonGaussian peak shapes (leads to additional band broadening plus need for new equations) Chromatographic Theory Questions on Definitions 1. List two ways in which a stationary phase is “attached” to a column? 2. What column component is present in packed columns but not open-tubular columns? 3. In HPLC, typical packing material consist of μm diameter spherical particles. Even though tightly packing the spheres should lead to > 50% of the column being sphere volume, the ratio of VM/Column Volume > 0.5. Explain this. Chromatographic Theory Questions on Definitions 4. 5. 6. List 3 main components of chromatographs. A chemist perform trial runs on a 4.6 mm diameter column with a flow rate of 1.4 mL/min. She then wants to scale up to a 15 mm diameter column (to isolate large quantities of compounds) of same length. What should be the flow rate to keep u (mobile phase velocity) constant? A chemist purchases a new open tubular GC column that is identical to the old GC column except for having a greater film thickness of stationary phase. Which parameters will be affected: KC, k, tM, tR(component X), β, a. Chromatographic Theory Questions on Definitions 7. What “easy” change can be made to increase KC in GC? In HPLC? 8. A GC is operated close to the maximum column temperature and for a desired analyte, k = 10. Is this good? 9. If a new column for problem 8 could be purchased, what would be changed? 10. In reversed-phase HPLC, the mobile phase is 90% H2O, 10% ACN and k = 10, is this good? 11. Column A is 100 mm long with H = 0.024 mm. Column B is 250 mm long with H = 0.090 mm. Which column will give more efficient separations (under conditions for determining H)? Chromatographic Theory Questions on Definitions 5.756 6.659 250 7.872 200 150 2.208 2.842 50 2.599 100 0 1 2 3 4 5 6 7 8 min 17.5 min ADC1 A, ADC1 CHANNEL A (LILLIAN\102507000009.D) VWD1 A, Wavelength=210 nm (LILLIAN\102507000009.D) ADC1 A, ADC1 CHANNEL A (LILLIAN\102507000006.D) VWD1 A, Wavelength=210 nm (LILLIAN\102507000006.D) 12.754 mV 1000 Unretained pk 1.204 1.201 14.242 800 600 400 0 2.5 5 7.5 12.5 15.436 12.821 8.309 8.444 10 14.103 0 7.173 200 2.696 2.695 – Which column shows a larger N value? – Which shows better resolution (1st 2 peaks top chromatogram)? – Which shows better selectivity (larger a; 1st 2 peaks on top)? – Should be able to calculate k, N, RS, and α mV 0.841 0.845 0.926 0.924 1.042 1.470 1.473 1.613 1.616 • Given the two chromatograms to the right: ADC1 A, ADC1 CHANNEL A (MONIQUE\062608000004.D) ADC1 B, ADC1 CHANNEL B (MONIQUE\062608000004.D) VWD1 A, Wavelength=205 nm (MONIQUE\062608000004.D) 15