Slide show - Cornell University
advertisement
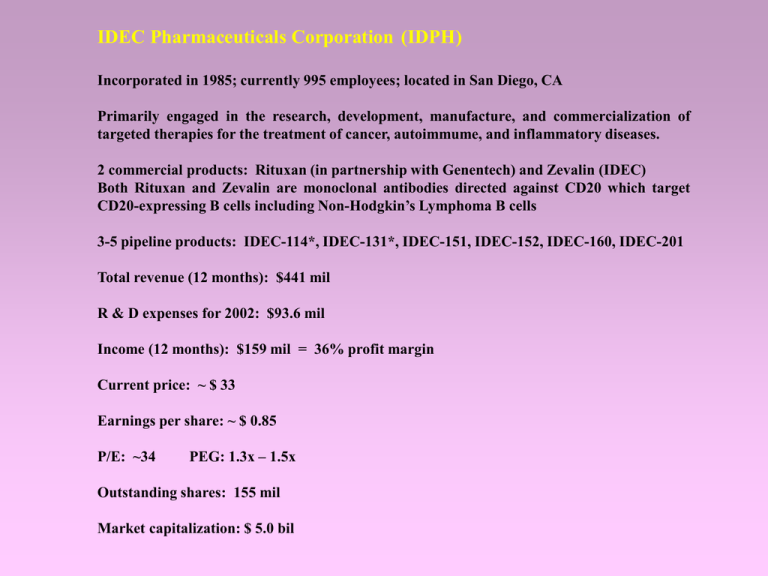
IDEC Pharmaceuticals Corporation (IDPH) Incorporated in 1985; currently 995 employees; located in San Diego, CA Primarily engaged in the research, development, manufacture, and commercialization of targeted therapies for the treatment of cancer, autoimmume, and inflammatory diseases. 2 commercial products: Rituxan (in partnership with Genentech) and Zevalin (IDEC) Both Rituxan and Zevalin are monoclonal antibodies directed against CD20 which target CD20-expressing B cells including Non-Hodgkin’s Lymphoma B cells 3-5 pipeline products: IDEC-114*, IDEC-131*, IDEC-151, IDEC-152, IDEC-160, IDEC-201 Total revenue (12 months): $441 mil R & D expenses for 2002: $93.6 mil Income (12 months): $159 mil = 36% profit margin Current price: ~ $ 33 Earnings per share: ~ $ 0.85 P/E: ~34 PEG: 1.3x – 1.5x Outstanding shares: 155 mil Market capitalization: $ 5.0 bil PROPRIETARY TECHNOLOGIES by IDEC PRIMATIZED Antibody Technology PRIMATIZED antibodies are chimeric antibody molecules consisting of the variable antigen-binding region from a mouse antibody fused to the constant region of a primate antibody (macaque). Primatized antibodies are structurally identical to human antibodies and reduce potential human-anti-mouse reactivity. IDEC was issued a US patent in 1998 for the development of PRIMATIZED antibodies. Macaque monkeys can also be immunized with human antigens to develop genetically engineered primatized antibodies which are produced in host cell lines. 3 product candidates currently under development utilize this technology. Proprietary Vector Technology IDEC has developed methods of engineering mammalian cell cultures using proprietary gene expression technologies or vector technologies that rapidly and reproducibly select for stable cells to produce high levels of desired proteins. This allows for efficient production of proteins at yields that are competitive with current commercial cell culture manufacturing methods, and has been used in the production of Rituxan. Fab Ag Ag Hybridoma Technology Fa IgL k,l IgH m,d,g,e,a Fb Fc A hybridoma is a cell line in which an antibody-producing B cell (human) is fused to an immortalized or long-lived mouse cell to produce a hybrid cell. The hybrid cell is cloned and expanded in culture to yield continuous production of an antibody of predetermined specificity. Industry-wide sales projections of monoclonal antibody therapeutics 16 14 $ Billions 12 10 8 6 4 2 0 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Credit Suisse First Boston Company Abgenix Amgen Biogen Corixa Genentech Glaxo-Smith Kline IDEC Imclone Johnson & Johnson Medarex Medimmune Xoma Zymogenetics Ticker ABGX AMGN BGEN CRXA DNA GSK IDPH IMCLE JNJ MEDX MEDI XOMA ZGEN Price 11.8 64.6 45.2 7.6 72.8 41.8 40.6 36.6 53.5 7.1 38.1 6.3 15.3 P/E n/a n/a 36.8 n/a 323.13 19.9 43.79 n/a 23.45 n/a 69.11 n/a n/a Market cap $ Mil 1,035 83,251 6,712 382 28,303 129,000 6,298 2,691 158,900 546 9,612 451 704 52 wk hi-lo 4.52 - 14.65 Ab-EGF 5.06 - 9.23 ENBREL (TNF inhibitor) 28.43 - 50.52 Avonex (IFN-1a for MS), Amevive (psoriasis) Bexxar (anti-CD20-I131) 7.52 - 7.76 Rituxan (anti-CD20) 25.10- 75.24 Bexxar (anti-CD20-I131) 31.35 - 43.87 Rituxan, Zevalin (anti-CD20-Yt90) 20.76 - 47.67 5.24 - 47.13 Erbitux (anti-EGFR) 41.40 - 61.30 Remicade (TNFR inhibitor) 2.55 - 9.00 UltiMab 20.37 - 42.09 Synagis (mAb blocks RSV infection) 2.84 - 8.00 Raptiva (anti-CD11a) 4.98 - 18.00 protein discovery via bioinformatics EXECUTIVE OFFICERS William H. Tastetter, Ph.D. Chairman, Chief Executive Officer (1986) William R. Rohn President, Chief Operating Officer (1993) Paul C. Grint, M.D. Senior Vice President, Chief Medical Officer (2001) Nabil Hanna, Ph.D. Senior Vice President, Chief Scientific Officer (1990) Wolfgang Berthold, Ph.D. Senior Vice President, Biopharmaceutical Sciences (2000) John M. Dunn Senior Vice President, Legal and Compliance, General Counsel and Corporate Secretary (2002) Connie L. Matsui Senior Vice President, Planning and Resource Devt (1992) Edward M. Rodiriguez Vice President, Finance and Controller (1991) Michael E. Wiebe, Ph.D. Vice President, Quality (2001) Mark C. Wiggins Vice President, Marketing and Business Devt (1998) Cash and Cash Equivalents = $1,447,865,000 Operating Expenses Dec 31, 2002 Dec 31, 2001 Dec 31, 2000 Research and Development $93,648,000 $86,299,000 $68,922,000 Selling, general, and Administrative (marketing $95,241,000 $55,241,000 $27,767,000 Cost of Revenue/ Manufacturing $1,457,000 N/A $2,134,000 Dec 31, 2002 Dec 31, 2001 Dec 31, 2000 Total Revenue $404,222,000 $272,677,000 $154,682,000 Net Income $148,090,000 (+45% from previous year, 37% profit)) $101,659,000 (+111% from previous year, 37% profit)) $48,145,000 (+11% from previous year, 31% profit) $0.85 per share (+44%) $0.59 per share (+96%) $0.30 per share (+0%) Revenues RITUXAN •Humanized anti-CD20 monoclonal antibody approved for NHL in November 1997; also being used off-label to treat CLL; first monoclonal Ab approved for treatment of cancer •Co-marketed by Genentech—sales reached $779 mil by 2001 and has steadily grown for the past four years ($152, $262, $424, $779 mil); IDEC retains approx. 40% of total sales; Estimate $1.38 bil in total sales for 2003; Expect 4-8% growth each quarter; $2500 per 500 mg dose •Estimated that 300,000 people in the US are afflicted with NHL (140,000 treatable with Rituxan) •Efficiency: Overall response rate of 48% with 6% complete response rate with a median time of 11.6 months •Achieved new approval in 2001 to expand its use in patients with relapsed or refractory, low-grade or follicular CD20+ B cell NHL, up to eight rather than four weekly infusions, and use of Rituxan in the treatment of bulky disease (tumors greater than 10 cm in diameter) •Approved in Japan for NHL co-promoted by Nippon-Roche and Zenayaku Kogyo •60 percent of patients undergoing treatment with Rituxan plus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) are still in remission after more than 4 to 7 years •Data from a phase III trial in Europe (GELA) indicate that patients with aggressive NHL treated with Rituxan plus CHOP show a 23 percent improvement in survival over patients treated with CHOP alone •Preclinical evidence that Rituxan may be useful for the treatment of autoimmune and inflammatory diseases, either alone or in combination with other drugs, including immune thromocytopenic purpura (ITP), systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA) Sales of Zevalin Price per Dose = $17,000 Sales to Date: • $13.7million since April 2002 • $5.7million in Q1 2003 Projections from S&P: • $68 million in 2003, • $118 million in 2004, • $200 million in 2007 ZEVALIN •Anti-CD20 (retuximab) non-humanized antibody coupled to yttrium-90 (beta-emitter) was approved for NHL in February 2002. IDEC has exclusive rights to this product in the US. Outside, Schering AG has exclusive marketing rights with IDEC receiving milestone payments and royalties. •Provides a new treatment option for patients when Rituxan or chemotherapy is not sufficient; concentrates the effect of radioimmunotherapy to the site of the tumor •Each treatment is complete in 8 days, but patient must make several hospital visits for treatment and imaging, and requires an integrated effort among the hospital community and physicians/oncologists using Zevalin to treat NHL disease. •Efficiency: 74% overall rate and 15% complete response (80% with Rituxan combined versus 56% Rituxan alone n=143) •CMS reimbursement rule (wage index adjustment) for Zevalin became effective in March 2003 has led roughly 24% of the hospitals to lose money with the Zevalin regimen. Expect no sales from these hospitals, thereby reducing the Zevalin sales estimates from $36 mil down to $27 mil. •Competes with Bexxar (Corixa/Glaxo-Smith-Kline) 131I-coupled anti-CD20 mAb (beta/gamma emitter); Bexxar produced a 70% overall response rate in low-grade NHL (35% complete response lasting a median of 3+ years n=251); 40% complete response rate in patients who are resistant to Retuximab treatment Charlotte Wolter, NHL patient, and Dr. Thomas Witzig, M.D., Hematologist, on Zevalin NEW PRODUCTS IN THE PIPELINE…. IDEC-114* (primatized anti-CD80 or anti-B7.1) This Ab selectively targets co-stimulatory B7-1 ligand on APCs. The neutralized molecule cannot bind to the CD28 and CTLA-4 receptors on T cells, thus blocking the second signal for inflammatory T-cell activation. • Two Phase II clinical trials for treatment of PSORIASIS (inflammation of skin affecting 1.5 Mil people in US) – DISCONTINUED due to lack of efficacy XOMA humanized anti-CD11a (Raptiva/Xanelim/Efalizumab) blocks T cell binding Developed and marketed by Genentech Psoriasis (BLA/MAA); Rheumatoid Arthritis, Psoriatic Arthritis (Phase II) RECENTLY HALTED CORIXA PVAC proprietary immunomodulator based on heat-killed Mycobacterium vaccae (Phase II) • Phase I/II clinical trial initiated in January 2002 for treatment of relapsed or refractory NON-HODGKINS LYMPHOMA and also in combination with Rituxan (estimated 300,000 patients in US have some form of NHL, 140,000 of which are lowgrade NHL or transformed low-grade NHL) NEW PRODUCTS continued…. IDEC-131* (anti-CD40L or anti-CD154) CD40L is a member of the TNFR family; expressed on activated T cells; provides costimulatory help to CD40-expressing B cells, macrophages, and dendritic cells. Ultimately reduce Ab production and restore normal immune response. • 1 Phase II clinical trial in PSORIASIS XOMA Raptiva CORIXA PVAC MEDAREX HuMax-CD4 (out-licensed to GENMAB) • 1 Phase II clinical trial in IMMUNE THROMBOCYTOPENIC PURPURA (anti-platelet disease) • 1 Phase II clinical trial in CROHN’S DISEASE (Inflammatory bowel disease) JOHNSON & JOHNSON/CENTOCOR Remicade (anti-TNF; approved) XOMA Neuprex rBPI21(Phase II) •1 physician-sponsored IND in MULTIPLE SCLEROSIS (demyelinating disease affecting 2.5 mil worldwide, 350,000 in US) CORIXA Anergix.MS (MHC class II complexed with MS peptide; Phase I/II done) * Clinical trials for IDEC-131 were suspended in 2002 due to safety concerns (thromboembolism), and are expected to resume in 2003 NEW PRODUCTS continued…. IDEC-151 (primatized anti-CD4) CD4 is expressed on thymocyte subsets and mature T cells; Helper T cells use CD4 to bind MHC class II. IDEC-151 is thought to prevent the helper T cell from becoming activated, without seriously affecting other immune system functions. •Completed a Phase II clinical trial n =32 in combination with methotrexate for RHEUMATOID ARTHRITIS (RA) (2 million people affected in the US and 1-2 percent worldwide); results promising JOHNSON & JOHNSON/CENTOCOR Remicade (anti-TNF) CORIXA Anergix.RA (MHC class II - collagen peptide complex; completed Phase I/II) Anervax.RA (peptide vaccine; completed Phase II) MEDAREX HuMax-CD4 and HuMax-IL-15 (outlicensed to GENMAB) AMGEN/WYETH Enbrel soluble TNFR (neutralizes TNF) ABBOTT rTNFR IDEC-152 (primatized anti-CD23) CD23 or FcReRII is a C-type lectin; low affinity receptor for IgE; expressed on mature B cells, activated macrophages, eosinophils, follicular DCs, and platelets •1 Phase I/II clinical trial initiated Feb 2002 n=30 for ALLERGIC ASTHMA and ALLERGIC RHINITIS (17 million people affected in the US); reduction in IgE levels but no observable reduction in clinical symptom scores •1 Phase I study initiated in Sep 2002 for CHRONIC LYMPHOCYTIC LEUKEMIA (30% of all leukemias, 7% NHLs, 8,100 new cases each year in US) NEW PRODUCTS continued…. IDEC-160 (HMN-214) This is a licensed, orally-administered small molecule developed by Nippon Shinyaku of Japan • 1 Phase I clinical trial initiated Sep 2002 (in US only) for treatment of solid tumor cancers • Shinyaku has a Phase I/II trial in and marketing rights Japan/Asia IDEC-201 (beta-interferon gene therapy) IDEC-201 is a beta-interferon gene therapy based on a replication-defective adenoviral vector. Licensing agreement signed in January 2003 to co-develop this drug with BIOGEN for treatment of GLIOMA (brain cancer affecting 36,000 people in the US); Biogen and IDEC share worldwide development and marketing rights on this product • Phase I/II clinical trial for treatment of GLIOMA ongoing OTHER TECHNOLOGIES AND TREATMENTS IN THE WORKS… DOMAIN-DELETED ANTIBODIES “Domain-deleted” antibodies have fragments of the constant region in the immunoglobulin heavy chain removed Coupled to yttrium-90, “domain-deleted” antibodies could potentially provide successful delivery of radioisotope to solid tumors such as prostate, ovarian, colon, and lung cancers Anticipate launching a pilot study with yttrium-90 labeled domain-deleted antibodies in 2003 CANCER VACCINES / THERAPY Currently collaborating with NCI to combine the prostate specific antigen PAGE-4 with IDEC’s patented PROVAX adjuvant technology in therapeutic vaccines for human clinical trials on prostate cancer (189,000 men in US affected with prostate cancer) MEDAREX MDX-010 (Anti-CTLA4) Phase I/II for prostate and melanoma Preclinical studies of IDEC-152 (anti-CD23) in the treatment of chronic lymphocytic leukemia (CLL). All CLL cells express CD23. Anticipate launching a Phase I/II trial with IDEC-152 on CLL this year PATENT/LEGAL ISSUES RITUXAN and ZEVALIN Lawsuits filed by GSK in May 1999 and Sept 2000 regarding patent infringement of Rituxan sales in US and Europe—settled by Genentech and GSK Lawsuits filed by GSK and Corixa in Sept 2001 in US and Europe regarding patent infringement of manufacture and sales of Zevalin—under appeals in Europe, settled in US through a lump-sum licensing agreement ANTI-CD40L Lawsuits filed by Dartmouth and Columbia for anti-CD40L—resolved??? Lawsuits filed by IDEC against Immunex for anti-CD40L patent infringement IMMUNOTHERAPY INDUSTRY: COMPARABLE COMPANIES Company Abgenix Amgen Biogen Corixa Genentech Glaxo-Smith Kline IDEC Imclone Johnson & Johnson Medarex Medimmune Xoma Zymogenetics Ticker ABGX AMGN BGEN CRXA DNA GSK IDPH IMCLE JNJ MEDX MEDI XOMA ZGEN Price 10.3 60.54 38.89 7.6 55.48 40.78 32.86 18.59 53.32 4.74 32.83 5.71 12.4 P/E n/a n/a 31.2 n/a 239.1 19.1 36.5 n/a 23.9 n/a 60.1 n/a n/a Market cap $ Mil 904 Ab-EGF 78,067 ENBREL (TNF inhibitor) 5,774 Avonex (IFN-1a for MS), Amevive (psoriasis) 383 Bexxar (anti-CD20-I131) 28,303 Rituxan (anti-CD20) 125,900 Bexxar (anti-CD20-I131) 5,096 Rituxan, Zevalin (anti-CD20-Yt 90) 1,369 Erbitux (anti-EGFR) 158,100 Remicade (TNFR inhibitor) 366 UltiMab 8,276 Synagis (mAb blocks RSV infection) 411 Raptiva (anti-CD11a) 570 protein discovery via bioinformatics POTENTIAL RISKS OF INVESTMENT 1) Slowing Rituxan sales growth: 95% of IDEC’s sales are dependent on Rituxan 2) Zevalin’s failure to gain NHL market share and failure to obtain approval in Europe 3) Competition if Corixa/GSK’s Bexxar is approved 4) Adverse ruling in patent litigation regarding Zevalin and Rituxan 5) Competitive drug pricing 6) Reliance on third-party vendors and contract manufacturers (e.g. Yttrium-90) 7) Limited sales and marketing experience 8) Uncertainties in new drug approvals and clinical trials 9) Healthcare reimbursement and reform (e.g. CMS categorized Zevalin as a procedure rather than a drug) 10) Product liability RHEUMATOID ARTHRITIS Rheumatoid Arthritis (RA) is a chronic, systemic, inflammatory disease that chiefly affects the synovial membranes of multiple joints in the body. RA is considered an autoimmune disease that is acquired and in which genetic factors appear to play a role. The presence of HLA-DR4 antibody in 70 percent of patients with RA lends support to the genetic predisposition to the disease. Rheumatoid Factor(s) (RF) are antibodies to IgG, and are present in 60-80 percent of adults with the disease. High titers of RF are usually associated with more severe and active joint disease, greater systemic involvement, and a poorer prognosis for remission. RA, as well as other autoimmune diseases, includes widespread immunologic and inflammatory alterations of connective tissue. The prevalence of the disease is 1-2 percent of the general population world-wide with 2 million people affected in the US. Females with RA outnumber males by a 3:1 margin. Onset of the disease in adults is usually between the ages of 40 to 60 years, although it can occur at any age. The etiology of RA is unknown. Viral or bacterial infections are suspected. Indolent NHL Follicular lymphoma comprises 20% of all non-Hodgkin's lymphomas and up to 70% of the indolent lymphomas. Most patients with follicular lymphoma are over age 50. Nodal involvement is most common, often accompanied by splenic and bone marrow disease. The median survival ranges from 8 to 12 yrs, thus designated "indolent." The vast majority of patients with advanced-stage follicular lymphoma are not cured with current therapeutic options. Rate of relapse is fairly consistent over time, even in patients who have achieved complete responses to treatment. Therapeutic options include purine nucleoside analogs, oral alkylating agents, combination chemotherapy, interferon, and monoclonal antibodies. Radiolabeled monoclonal antibodies, vaccines, and autologous or allogeneic bone marrow or peripheral stem cell transplantation are under clinical evaluation. Rearrangement of the bcl-2 gene is present in over 90% of patients with follicular lymphoma; over-expression of the bcl-2 protein is associated with the inability to eradicate the lymphoma by inhibiting apoptosis. Aggressive NHL Diffuse large B-cell lymphoma is the most common of the non-Hodgkin's lymphomas, comprising 30% of newly-diagnosed cases. Most patients present with rapidly enlarging masses, often with symptoms both locally and systemically. The vast majority of patients with localized disease are curable with combined modality therapy. For patients with advanced stage disease, 40% of presenting patients are cured with doxorubicin-based combination chemotherapy. An international index for aggressive NHL (diffuse large cell lymphoma) identifies 5 significant risk factors prognostic of overall survival: age (<60 years of age), serum lactate dehydrogenase, stage (I or II versus III or IV), and extranodal site involvement (0 or 1 versus 2-4). Patients with 2 or more risk factors have less than a 50% chance of relapse-free and overall survival at 5 years. This study also identifies patients at high risk of relapse based on specific sites of involvement, including bone marrow, central nervous system (CNS), liver, lung, and spleen. A retrospective analysis of 605 patients with diffuse large cell lymphoma who did not receive Some cases of large B-cell lymphoma have a prominent background of reactive T cells and often of histiocytes, so-called T-cell/histocyte-rich large B-cell lymphoma. This subtype of large cell lymphoma has a very aggressive clinical course with frequent liver, spleen, and bone marrow involvement. Non-Hodgkin’s Lymphoma Classifications Low-grade A Small lymphocytic, CLL (SL) B Follicular, small cleaved cell (FSC) C Follicular, mixed small cleaved and large cell (FM) Intermediate-grade D Follicular, large cell (FL) E Diffuse, small cleaved cell (DSC) F Diffuse mixed, small and large cell (SM) G Diffuse, large cell cleaved or noncleaved (DM) High-grade H Immunoblastic, large cell (IBL) I Lymphoblastic, convoluted or non-convoluted (LL) J Small noncleaved cell, Burkitt’s or non-Burkitt’s (SNC) History of Rituxan and Zevalin Rituxan (Rituximab): a primatized MCA directed against CD20 • approved for use in refractory low-grade or follicular, CD20+, B-cell non-Hodgkin’s lymphoma in 1997--44,000 cases/year • first monoclonal antibody approved for cancer therapy • largest selling oncology product in the world-and it may grow as variations for its use are studied and adopted (e.g., it is being used and tested as a Maintenance therapy) • good preliminary data supports its use in rheumatoid arthritis and systemic lupus and phase 3 trials are in progress Zevalin (Ibritumomab tiuxetan): the same MCA conjugated to chelating agent that binds either a beta or gamma emitter (for therapy or imaging) • approved for treatment of relapsed or refractory low grade (to chemo and Rituxan), follicular, or transformed B-cell non-Hodgkin's lymphoma (NHL) in February 2002 • first radioimmunotherpay approved for cancer therapy Sales of Rituxan Two-tier copromotion arrangement with Genentech + royalties from non-US sales (from Roche) in millions Q1 Q2 Q3 Q4 Year Total 2003 $341.0 2002 $247.5 $274.9 $293.9 $346.6 $1,162.9 (385,809) 2001 $172.1 $187.7 $212.8 $246.0 $818.6 (251,428) 2000 $85.1 $102.8 $117.9 $138.3 $444.1 (132,782) 1999 $57.1 $74.4 $72.6 $75.3 $279.4 1998 $37.7 $34.8 $39.4 $50.6 $162.6 1997 - - - $5.5 $5.5 ( ) = Copromotion Profits + royalties from non-US sales Sales of Zevalin IDEC holds 100% marketing rights within the US and signed an agreement with Schering AG for outside US which pays milestones and royalties IDEC only recently started getting reimbursed so revenues are just beginning to be seen 2002 since April (13.7 million), 2003 Q1(5.7 million) Future of Rituxan • Rituxan sales decreased during the past quarter - is the market saturated or does this reflect the price increase?? • Many clinical trials exploring Rituxan as treatment in other diseases - would IDEC retain marketing rights for this label? • Competition Projections S&P $1.29billion in 2003; $1.44billion in 2004 But this ignores use for other indications Rheumatoid arthritis • a chronic condition • 1 percent of the population (2/3 women) • ‘overactive’ immune system attacks joints and causes crippling pain • Current therapy are: anti-T cells or anti inflammatory drugs • Rituxan is the only B cells directed therapy, but there is a controversy about the importance of B cells in the disease • Only minor side effects in preliminary studies and it is already and FDA approved Drug • So…... Efficacy and Safety of Rituximab,a B-Cell Targeted Chimeric Monoclonal Antibody: A Randomized, PlaceboControlled Trial in Patients with Rheumatoid Arthritis (Edwards et al: Arthritis Rheum. 2002 Sep;46(9):S197. Abstract # 446. Other Autoimmune Diseases effectiveness in one patient with refractory type II essential mixed cryoglobulinemia (MC) and one with refractory Goodpasture's syndrome Treatment of Systemic Lupus (SLE) is promising complete remission in a patient with refractory Wegener's granulomatosis dramatic and sustained improvement" in five patients with refractory adult or pediatric dermatomyositis Future of Zevalin •Direct Competition: Feb 23, 2003 infringement suit filed against Corixa and GSK for Bexxar, a CD20 specific radioligated antibody Projections: •$68 million in 2003, • $118 million in 2004, •$200 million in 2007 •Additional studies may create new uses (other disorders or primary treatment) Existing products: Conclusions • Use of existing products for slight variations in the cancer area should continue to increase sales for several years • Sales are already being driven by some off label use • Preliminary data suggests approval for RA and related uses will occur • This could double (optimistically) the top line for existing products, lowering P/E to below 20?? or increasing the price of the stock?? IDEC Patents Combination therapies for B-cell lymphomas comprising administration of anti-CD20 antibody Granted US and European patent protection PRIMATIZED Antibody Technology Chimeric antibody molecules consisting of the variable antigen-binding region from mouse antibodies fused to the constant region of primate antibodies (macaque). Primatized antibodies are structurally identical to human antibodies and reduce potential human-anti-mouse reactivity. Granted worldwide patent protection: covers antibody technology as well as specific antigen targets of PRIMATIZED antibodies Method for integrating genes at specific sites in mammalian cells via homologous recombination and vectors for accomplishing the same Methods of engineering mammalian cell cultures using proprietary gene IDEC literature Zevalin and Rituxan: Clinical Trials Additional Radiation Absorbed Dose Estimates for Zevalin trade mark Radioimmunotherapy. Cancer Biother Radiopharm. 2003 Apr;18(2):253-8. Wiseman GA, Leigh BR, Dunn WL, Stabin MG, White CA. Mayo Clinic, Rochester, MN. Radiation Dosimetry Results From a Phase II Trial of Ibritumomab Tiuxetan (Zevalin trade mark ) Radioimmunotherapy for Patients With Non-Hodgkin's Lymphoma and Mild Thrombocytopenia. Cancer Biother Radiopharm. 2003 Apr;18(2):165-78. Wiseman GA, Leigh BR, Other: Notch-regulated ankyrin-repeat protein inhibits notch1 signaling: m signaling pathways involved in T cell development. J Immunol. 2003 Jun 15;170(12):5834-41. Yun TJ, Bevan MJ. IDEC Ph San Diego, CA 92121. Department of Immunology and Howard Hughes University of Washington, Seattle, WA 98195. CD154 Regulates Primate Humoral Immunity to Influenza. Am J Transplant. 2003 Jun;3(6):680-688. Crowe JE, Sannella EC, Pfeiff Azimzadeh A, Newman R, Miller GG, Pierson RN. Departments of Ped Microbiology and Immunology, Cardiothoracic Surgery, and Medicine, University Medical Center, Nashville, TN, USA IDEC Pharmaceuticals, USA Nashville Veterans Affairs Medical Center, Nashville, TN, 37232,







