Do Now - Glowzenski
advertisement

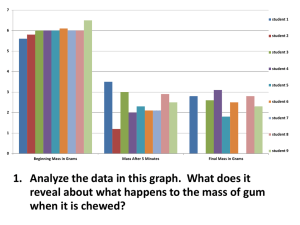
Physical Science Do Now February 24th 2011 Matter: anything that has mass and takes up space Mass – the amount of matter in something Volume – the amount of space something occupies Answer the following four questions: 1) What is an example of solid matter? 2) What is an example of liquid matter? 3) What is an example of gaseous matter? 4) What is an example of something that is NOT matter? SWBAT calculate basic density problems HOMEWORK: Section Review Page 52 Numbers 1 6 Do Now Review! 1) What is an example of solid matter? 1) 2) 3) 4) Box Person Car Desk 1) 2) 3) 4) 5) Water Oil Juice Soda Liquid Nitrogen 1) 2) 3) 4) Air Oxygen gas Nitrogen gas Methane 1) Light, is not matter!!, it is simply a reflection of light off of the gaseous air particles! 2) What is an example of liquid matter? 3) What is an example of gaseous matter? 4) What is an example of something that is NOT matter? What is a property? • Property: a characteristic of a substance that can be observed • What do you observe about this clip art? Physical Property Physical property: a property that can be observed without changing the identity of the substance. Examples: • luster • melting point • malleability: the ability to be hammered into a thin sheet • density • ductility: the ability to be stretched into a wire • boiling point • solubility • specific heat Special Physical Properties • Melting point: the temperature at which a substance changes from a solid to a liquid at a given pressure water = 0oC • Boiling point: the temperature at which a substance changes from a liquid to a gas at a given pressure water = 100oC Chemical Properties • Chemical property: a property that can only be observed by changing the identity of the substance Examples: •flammability •ability to rust •reactivity with vinegar Density • Density is the amount of mass per unit of volume. • Density can be used to identify a substance. • The density of water is 1.0g/mL Density Calculations • Calculations: – Density = Mass / Volume – Density = Mass Volume • Ex: A cube has a mass of 4.0 g and occupies a volume of 2.0 ml. What is the DENSITY • Density = Mass / Volume • Density = 4.0 g / 2.0 ml • Density = 2.0 g/ml More Density Calculations • Ex: A liquid has a mass of 25.0 g and a volume of 5.0 mL. • Density = Mass / Volume • Density = 25.0 g / 5.0 ml • Density = 5.0 g/ml Substance Density (g/mL) Mercury 13.6 Water 1.00 Ethanol 0.81 Try these density problems in notebook… 1) 100 grams of a liquid completely fill a 200 mL bottle. What is the density of the liquid? 2) A scientist puts 50 grams of a substance into a 100mL flask. What is the density of the liquid? 3) A piece of silver has a mass of 2800 grams and occupies a volume of 266 mL. What is the density of silver? 4) A student pipets 5.00 mL of ethanol into a flask weighing 15.25 grams. Calculate the density of ethyl alcohol 5) A block of copper weighs 453 g and is placed into a solution occupying a volume of 525 mL, what is the density? Review 1) 100 grams of a liquid completely fill a 200 mL bottle. What is the density of the liquid? 1) D = mass/volume = 100g / 200mL = 0.50 g/mL 2) A scientist puts 50 grams of a substance into a 100mL flask. What is the density of the liquid? 1) D = mass/volume = 50g / 100mL = 0.50 g/mL 3) A piece of silver has a mass of 2800 grams and occupies a volume of 266 mL. What is the density of silver? 1) D = mass/volume = 2800g / 266mL = 10.53 g/mL 4) A student pipets 5.00 mL of ethanol into a flask weighing 15.25 grams. Calculate the density of ethyl alcohol ? 1) D = mass/volume = 15.25g / 5.00mL = 3.05 g/mL 5) A block of copper weighs 453 g and is placed into a solution occupying a volume of 525 mL, what is the density? 1) D = mass/volume = 453g / 525mL = 0.86 g/mL Homework • HOMEWORK: Section Review • Page 52 • Numbers 1 6 Achieve 3000 • Log on to: www.achieve3000.com • Read the homepage article “Soccer Star Keeps Giving” • Read the mail article under the subject: Physical Science 2/21 – “Turn it Down” • HOMEWORK: Section Review • Page 52 • Numbers 1 6