Heat Absorbed by Ice Heat Transferred from Water
advertisement

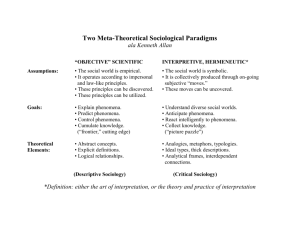
ISNS 4371 - Phenomena of Nature The state or phase of matter is determined by its temperature. Consider water: Below 32º F - ice - relatively low KE - each molecule tightly bound to it neighbors - solid At 32º F molecules have enough energy to break solid bonds of ice - remain together but move relatively freely - liquid At 212º F water boils and turns to gas - molecules break free of all bonds with neighbors - move independently of other molecules - gas ISNS 4371 - Phenomena of Nature Phase/State Changes Heat transfer always occurs whenever a substance changes phase Melting - when a solid changes to a liquid Energy is absorbed Evaporation - when a liquid changes to a gas Sublimation - when a solid changes directly to a gas Energy is released Condensation - when a gas changes to a liquid Freezing - when a liquid changes to a solid ISNS 4371 - Phenomena of Nature Change of State (Phase Change) Latent heat of fusion - amount of thermal energy required to change a substance from a solid to a liquid (melting). Latent heat of solidification - amount of thermal energy given up when a substance changes from a liquid to a solid (freezing). Latent heat of fusion for water is 80 calories per gram. Latent heat of solidification for water is -80 calories per gram. Energy is released. ISNS 4371 - Phenomena of Nature Change of State (Phase Change) Latent heat of vaporization - amount of thermal energy required to change a substance from a liquid to a gas (vaporization or evaporation). Latent heat of condensation - amount of thermal energy given up when a substance changes from a gas to a liquid. (condensation). Latent heat of vaporization for water is 539 calories per gram. Latent heat of condensation for water is -539 calories per gram. Energy is released. ISNS 4371 - Phenomena of Nature Phase Change Diagram ISNS 4371 - Phenomena of Nature Specific Heat Capacity, c: Thermal inertia Specific Heat Capacity is the quantity of heat required to change the temperature of 1 gram of a substance by 1° C. Q units of of thermal energy added to 1 gram of a substance produces a temperature change of ∆T, Q = c x ∆T Specific heat , c, of a substance is the heat capacity per unit mass. For m grams of a substance, Q = cm ∆T or ∆T = Q/cm Water has high specific heat capacity - used as a cooling fluid. Specific heat capacity of water is 1 calorie/gram-deg. C ISNS 4371 - Phenomena of Nature Heat of Fusion Measurement Add 10 grams of ice (at 0º C) to 100 grams of water. What is the heat of fusion of water? Mass of water=M Mass of ice =m Hf= heat of fusion of water To = initial temperature of ice Tw = initial temperature of water Tf = final temperature of water Heat required to melt the ice = mHf Heat required to raise the temperature of melted ice to final temperature of water = cm ∆T = cm(Tf - To) Heat absorbed from water = cM ∆T = cM(Tw-Tf) ISNS 4371 - Phenomena of Nature Heat of Fusion Heat Absorbed by Ice Heat Transferred from Water Q1 = cm(Tf - To) +mHf Q2 = cM(Tw - Tf) Equate heat absorbed by ice to heat transferred from water cm(Tf - To) + mHf = cM(Tw - Tf) To = 0 and c = 1, so mHf = M(Tw - Tf) - m Tf Hf = M/m((Tw - Tf) - Tf ISNS 4371 - Phenomena of Nature Heat of Fusion Heat Absorbed by Ice Heat Transferred from Water Q1 = cm(Tf - To) +mHf Q2 = cM(Tw - Tf) Equate heat absorbed by ice to heat transferred from water cm(Tf - To) + mHf = cM(Tw - Tf) To = 0 and c = 1, so mHf = M(Tw - Tf) - m Tf Hf = M/m((Tw - Tf) - Tf Hf = 80 cal/gr ISNS 4371 - Phenomena of Nature Change of State (Phase Change) Example: Add 10 grams of ice at 0º C to 100 grams of water at 30 º C. What is the final temperature of the water? Heat Absorbed by Ice Heat Transferred from Water Mass of ice =m Mass of water=M Hf= heat of fusion of water: 80 calories/gram To = initial temperature of ice Tw = initial temperature of water Tf = final temperature of water Q=cm(Tf - To) +mHf Q=cM(Tw-Tf) ISNS 4371 - Phenomena of Nature Change of State (Phase Change) Heat Absorbed by Ice Heat Transferred from Water Mass of ice = m = 10 grams Mass of water = M = 100 grams Hf= heat of fusion of water: 80 calories/gram Q = cm(Tf - To) +mHf Q = cM(Tw - Tf) Q = 1 x 10(Tf - 0) + 10 x 80 Q = 1 x 100(30 - Tf) Equate heat absorbed by ice to heat transferred from water 10Tf + 800 = 3000 - 100Tf 110Tf = 3000 - 800 Tf = 2200/110 ISNS 4371 - Phenomena of Nature Change of State (Phase Change) Heat Absorbed by Ice Heat Transferred from Water Mass of ice = m = 10 grams Mass of water = M = 100 grams Hf= heat of fusion of water: 80 calories/gram Q = cm(Tf - To) +mHf Q = cM(Tw - Tf) Q = 1 x 10(Tf - 0) + 10 x 80 Q = 1 x 100(30 - Tf) Equate heat absorbed by ice to heat transferred from water 10Tf + 800 = 3000 - 100Tf 110Tf = 3000 - 800 Tf = 2200/110 Tf = 20º C ISNS 4371 - Phenomena of Nature Atmospheric Pressure Atmosphere - layer of gas surrounding a world Atmospheric pressure - collisions of individual atoms or molecules in atmosphere Air molecules in a balloon exert pressure as they collide with the walls pushing outward. Air molecules outside balloon collide with wall and exert pressure inward. Balloon stays inflated when pressures are balanced. Adding molecules to balloon (blow it up) causes balloon to expand (increases its volume) until pressures are balanced again. Heating it also increases pressure (increases the speed of the molecules). The balloon expands until pressures are equalized again ISNS 4371 - Phenomena of Nature Gas in an atmosphere is held down by gravity. Atmosphere above presses downward, compressing atmosphere below. At the same time, fast moving molecules exert pressure in all directions, including upward tends to make atmosphere expand. Planetary atmospheres exist in balance between downward weight of their gases and upward push of their gas pressure The higher you go, the less the weight of gas above you, and the less the atmospheric pressure. 1 bar - atmospheric pressure at sea level equal to weight of a column of gas extending upward from Earth’s surface from sea level ISNS 4371 - Phenomena of Nature Vapor Pressure and Boiling Point Evaporation in a closed container will proceed until there are as many molecules returning to the liquid from the vapor above the liquid as there are escaping - the vapor is then said to be saturated. The pressure of that vapor is called the saturated vapor pressure. Molecular kinetic energy is greater at higher temperature - more molecules can escape the surface and the saturated vapor pressure is correspondingly higher. If the liquid is open to the air, then the pressure of the air opposes the escape of the molecules. The temperature at which the vapor pressure is equal to the atmospheric pressure is called the boiling point. ISNS 4371 - Phenomena of Nature Evaporation vs Boiling Both start with a liquid and end with a gas. But they are different processes. Evaporation: Strictly a surface phenomena Occurs at any temperature Some hotter (faster)-than-average particles overcome the forces they feel from their neighbors and escape the liquid, taking their heat energy with them. Forces only felt from particles beneath them Boiling: Happens throughout the liquid Occurs at the boiling point/temperature Average motion of particles is fast enough to overcome the forces holding them close together - all the particles are trying to escape liquid turns to vapor Forces felt from particles all around them Boiling point dependent on atmospheric pressure - steam bubbles form in liquid only when vapor (steam) pressure exceeds atmospheric pressure (plus pressure of water pushing down) ISNS 4371 - Phenomena of Nature The Boiling Point Depends on the Liquid Temperature and the Atmospheric Pressure Boiling (evaporation) cools the liquid - when 100º C water is boiling, it is in thermal equilibrium - it is being cooled by the boiling as fast as it is being heated by the heat source - if not the water temperature would continue to rise ISNS 4371 - Phenomena of Nature Carbon Dioxide People breathe in oxygen and breathe out carbon dioxide. Plants breathe in carbon dioxide and breathe out oxygen. Carbon dioxide gas doesn’t turn into a liquid when it gets cold like most other matter, it turns straight into a solid - it sublimates - at -109° F! Sublimate - to transform directly from the solid to the gaseous state or from the gaseous to the solid state without becoming a liquid. Frozen carbon dioxide is called dry ice. We can make fog with dry ice by putting it in hot water. - the extreme cold causes the hot water vapor to condense into clouds - the dry ice turns into carbon dioxide gas and mixes with the clouds - the fog is heavier than air and sinks to the floor ISNS 4371 - Phenomena of Nature Nitrogen Most of the air we breathe ( 78%) is made of Nitrogen. Nitrogen gas turns into a liquid at -320° F and freezes at -346° F. We can use liquid nitrogen (N2) to freeze things. Some things become very hard when they are frozen - like a banana, some become very brittle - like a flower. The water in them freezes, forming millions of tiny ice crystals. The banana has fibers in it that hold the crystals together making it hard. The flower doesn’t have such fibers and becomes brittle. ISNS 4371 - Phenomena of Nature Freezing Nitrogen When we pump the air out of a container with liquid nitrogen in it, we make a vacuum above the liquid nitrogen. - the nitrogen starts to boil - because of a reduction in atmospheric pressure - reduction in boiling point - evaporation increases - heat for evaporation comes from liquid nitrogen (remember heat of vaporization) - N2 molecules are not returning to liquid because they are being removed by vacuum pump - temperature continues to drop - nitrogen freezes (quickly and easily in demonstration because temperature difference between liquid and solid N2 is only 26° F) Same process by which evaporation of sweat cools your skin or condensation of steam heats (burns) your skin Does the nitrogen ice float or sink? Its solid form is more dense than its liquid form - it sinks. ISNS 4371 - Phenomena of Nature Water, Wonderful Water ISNS 4371 - Phenomena of Nature Water is unusual because it is less dense as a solid than as a liquid. Most materials contract as they solidify, but water expands. At temperatures above 4oC, water behaves like other liquids, expanding when it warms and contracting when it cools. Ice is about 10% less dense than water at 4oC. ISNS 4371 - Phenomena of Nature Oceans and Lakes Don’t Freeze Solid Because Ice Floats Ice floats on the cool water below - has important consequences for life. – If ice sank, eventually all ponds, lakes, and even the ocean would freeze solid. – During the summer, only the upper few inches of the ocean would thaw. – Instead, the surface layer of ice insulates liquid water below, preventing it from freezing and allowing life to exist under the frozen surface. ISNS 4371 - Phenomena of Nature Water and the Environment Water has a high heat capacity - resists changes in temperature Compare with: ethyl alcohol - 0.6 cal/gr-deg iron - 0.1 cal/gr-deg Can absorb or release relatively large amounts of heat with only a slight change in its own temperature. Moderates temperatures on Earth - stabilizes air temperatures by absorbing heat from warmer air and releasing heat to cooler air. ISNS 4371 - Phenomena of Nature The impact of water’s high specific heat ranges from the level of the whole environment of Earth to that of individual organisms. – A large body of water can absorb a large amount of heat from the sun in daytime and during the summer, while warming only a few degrees. – At night and during the winter, the warm water will warm cooler air. – Therefore, ocean temperatures and coastal land areas have more stable temperatures than inland areas. – The water that dominates the composition of biological organisms moderates changes in temperature better than if composed of a liquid with a lower specific heat. ISNS 4371 - Phenomena of Nature Water and Weather Very important for weather Land cools and heats up more quickly than water Causes on shore/off wind circulation patterns Weather in northern hemisphere more extreme - more land mass - greater extremes in temperature Weather on US west coast less extreme than on east coast - prevailing winds blow west to east - blow over ocean in west and over land in east - greater extremes in temperature in east Water temperature also colder on west coast because of ocean currents