Enthalpy and thermochemical equations
advertisement

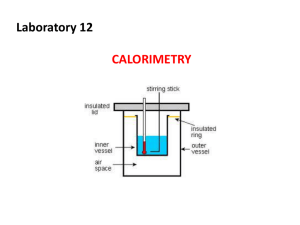
ENTHALPY, HESS’ LAW, AND THERMOCHEMICAL EQUATIONS Quick Review of Concepts We have been introduced to heat producing (exothermic) reactions and heat using (endothermic) reactions Heat is a measure of the transfer of energy from a system to the surroundings and from the surroundings to a system Enthalpy The heat given off or absorbed during a reaction (change in heat of a system) is called the change in enthalpy (ΔH) when the pressure of the system in kept constant DH = H (products) – H (reactants) More on Enthalpy Hproducts < Hreactants DH < 0 Hproducts > Hreactants DH > 0 6.3 Calorimetry We measure the transfer of heat (at a constant pressure) of a chemical reaction by a technique called calorimetry In calorimetry: The heat released by the system is equal to the heat absorbed by its surroundings The heat absorbed by the system is equal to the heat released by its surroundings The total heat of the system and the surroundings remains constant First Law of Thermodynamics Calorimetry We use an insulated device called a calorimeter to measure this heat transfer A typical device is a “coffee cup calorimeter” Reaction is open to the atmosphere Therefore, q constant pressure = ∆H at constant pressure Calorimetry To measure ΔH for a reaction using a coffee-cup calorimeter: 1. Dissolve the reacting chemicals in known volumes of water 2. Measure the initial temperatures of the solution 3. Mix the solutions 4. Measure the final temperature of the mixed solution Calorimetry The heat generated by the reactants is absorbed by the water We know the mass of the water, mwater We know the change in temperature, ∆Twater We also know that water has a specific heat of Cwater = 4.18 J/°C-g. We can calculate the heat of reaction by: qsys = ∆H = −qsurr qrxn = ∆H = −qwater qrxn = ∆H = -(mwater × Cwater × ∆Twater) Practice Problem • When 25.0 mL of water containing 0.025 mol of HCl at 25.0°C is added to 25.0 mL of water containing 0.025 mol of NaOH at 25.0°C in a coffee cup calorimeter, a reaction occurs. Calculate ∆H (in kJ) of one mole of water during this reaction if the highest temperature observed is 32.0°C. • Note: • • Assume the densities of the solution are 1.00 g/mL Assume all heat was absorbed by water Summary Heat is a measure of the transfer of energy from a system to the surroundings and from the surroundings to a system The change in heat of a system is called the change in enthalpy (ΔH) when the pressure of the system in kept constant We measure the transfer of heat (at a constant pressure) of a chemical reaction by a technique called calorimetry We use an insulated device called a calorimeter to measure this heat transfer Thermochemical Equations Is DH negative or positive? System absorbs heat Endothermic DH > 0 H2O (s) H2O (l) DH = + 6.01 kJ 6.3 Thermochemical Equations Is DH negative or positive? System gives off heat Exothermic DH < 0 CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) DH = -890.4 kJ Thermochemical Stoichiometry Enthalpy is STOICHIOMETRIC! Recall that the stoichiometric coefficients always refer to the number of moles of a substance H2O (s) H2O (l) DH = 6.01 kJ You would read the above equation as: “6.01 kJ are absorbed for every 1 mole of ice that melts at 00C and 1 atm” CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) DH = -890.4 kJ “890.4 kJ are released for every 1 mole of methane that is combusted at 250C and 1 atm” Practice Problem How much heat is evolved when 266 g of white phosphorus (P4) burn in air? P4 (s) + 5O2 (g) 266 g P4 x 1 mol P4 123.9 g P4 P4O10 (s) DH = -3013 kJ x 3013 kJ = 6470 kJ 1 mol P4 6.3 Let’s Try Some More! Page 165 Manipulating ∆H • If you reverse a reaction, the sign of DH changes H2O (l) • H2O (s) DH = -6.01 kJ If you multiply both sides of the equation by a factor n, then DH must change by the same factor n 2H2O (s) 2H2O (l) DH = 2 x 6.01 = 12.0 kJ