Dr. Vincent's PPT
advertisement

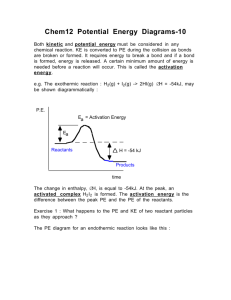
Using Chemical Demonstrations to Demonstrate Concepts in Physical Science II Holt Science & Technology: Physical Science Chapter 14 Chemical Reactions Section 4 Energy and Rates of Reactions Section Outline • Reactions and Energy - Exothermic reactions Figure 1. Types of Chemical Reactions -Law of Conservation of Energy Figure 2. No title • Rates of Reaction - Activation energy Figure 3. No title -Sources of Activation Energy Figure 4. Energy Diagrams Chapter 14 Chemical Reactions Section 4 Energy and Rates of Reactions Section Outline (cont.) • Factors Affecting Rate of Reaction - Temperature Figure 5. No title - Concentration Figure 6. Concentration of solutions - Catalyst Figure 7. No title Reactions and Energy • Chemical energy is part of all chemical reactions. – Energy is needed to break chemical bonds in reactions. – Energy is released when new chemical bonds form in chemical reactions. Reactions and Energy • Exothermic reaction – a chemical reaction that releases energy – Exo means “go out” or “exit” • Endothermic reaction – a chemical reaction that takes energy in – Endo means “go in” Demonstration 1 – Energy Release • Types of Energy release – Light – Electrical energy – Light and thermal energy Demonstration 1 – Light • Snapping a glowstick breaks a small glass container in the glowstick, allowing chemicals in the glass container to mix with chemicals outside the glass (but inside the chamber of the glowstick). • The resulting reaction gives off light. Demonstration 2 – Electrical Energy • Electrical energy is released in an exothermic reaction that takes place in the battery • See if the batteries get warm. 2 MnO2(s) + 2 H2O(l) + Zn(s) 2 MnO(OH)(s) + Zn(OH)2(s) Demonstration 3 – Heat and Light Release • Energy is released during some chemical reactions. The fire of a Bunsen burner gives off light energy and chemical energy. (An alcohol burner, candle, lighter, etc. could also be used, although reaction would change.) CH4 + 2 O2 CO2 + 2 H2O Signs of Chemical Reactions • • • • Gas formation Solid (precipitate) formation Color change Energy change – Light – Thermal energy (heat) – Electrical energy Law of Conservation of Energy • Law of conservation of energy – energy cannot be created or destroyed • Energy can change forms Energy can be transferred from one object to another in the same manner that a baton is transferred from one runner to another in a relay race. Rates of Reaction • Activation energy – smallest amount of energy that molecules need to react. Chemical reactions need energy to get started In the same way that a bowling ball needs a push to get rolling. Activation Energy Reactants Energy given off Reaction progress Energy Energy Activation energy Products Activation energy Energy absorbed Reaction progress Factors Affecting Rates of Reaction Demonstration 4 – Glow Sticks • Temperature – A higher temperature causes a faster reaction rate. – At higher T, particles of reactants move rapidly. – Rapid movement causes particles to collide often and with a lot of energy. – Many particles have the activation energy to react and change into products in a short time. The light stick on the right glows brighter than the one on the left because the one on the right is warmer. Factors Affecting Rates of Reaction • Concentration – In general, a high concentration of reactants causes a fast rate of reaction. – Concentration is a measure of the amount of one substance dissolved in another substance. – When the concentration is high, there are many reactant particles in a given volume and a small distance between them. – Thus, the particles run into each other often, leading particles to react faster. Factors Affecting Rates of Reaction • Surface Area • Surface area is the amount of exposed surface of a substance • Increasing surface area increases rate of reaction. • Greater surface area exposes more particles of the reactant to other reactant particles. • These leads to more collisions and faster rates. Demonstration 5 – Mentos and Diet Coke http://www.eepybird.com/featured-video/the-extreme-diet-coke-mentos-experiments-iithe-domino-effect// Factors Affecting Rates of Reaction • Inhibitors – An inhibitor is a substance that slows down or stops a reaction • Food preservatives – slow down growth of bacteria and fungi Factors Affecting Rates of Reaction • Catalysts – Catalyst – speeds up a chemical reaction without being consumed/ – A catalyst is not consumed because it is not a reactant. – A catalyst lowers the activation barrier, which allows a reaction to happen more quickly. This catalytic converter contains platinum and palladium. These two catalysts increase the rate of reactions that make the car’s exhaust less harmful. Demonstration 6 – Catalyst • Hydrogen peroxide, H2O2, is unstable but decomposes very slowly. The decomposition reaction gives off heat. If Cu2+ ions are added (as a catalyst) to add aqueous solution of H2O2, the reaction proceeds rapidly and can become hot enough to boil the water. • If dishwashing detergent is added to the solution before the catalyst, then a version of the elephant toothpaste demonstration is produced. 2 H2O2 O2 + 2 H2O Demonstration 7 – Clock Reaction • Concentration, temperature, inhibitor Demonstration 8 – Alka Seltzer • Concentration, temperature, surface area