Atomic X-Ray Spectrometry
advertisement
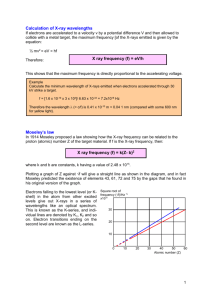
Instrumental Chemistry Chapter 12 Atomic X-Ray Spectrometry Brief Summary X-ray spectroscopy is a form of optical spectroscopy that utilizes emission, absorption, scattering, fluorescence, and diffraction of Xray radiation About X-Rays • X-rays are short-wavelength (hence, high frequency, and hence, relatively high energy) electromagnetic radiation. Two ways to produce X-rays: 1) Deceleration of high-energy electrons 2) Electronic transitions involving inner-orbital (e.g. d or f) electrons • The wavelength range of X-Rays is from about 10-5 Å to 100Å • Conventional X-Ray spectroscopy is largely confined to the region of about 0.1 Å to 25 Å For Analytical Purposes, X-rays are Generated in Three Ways: 1) Bombardment of metal target with high-energy electron beam 2) Exposure of target material to primary X-ray beam to create a secondary beam of X-ray fluorescence 3) Use of radioactive materials whose decay patterns include X-ray emission Schematic of an X-ray tube Energy-level iagram showing common transitions producing X-rays Energy-Level Diagram Showing Common Transitions Producing X-rays Common X-Ray Transitions • Partial energy level diagram showing common transitions leading to X– radiation. The most intense lines are indicated by the widest arrows Wavelengths/Å for Intense X–ray Emission Lines Note that all possible electronic transitions are not of equal probability, i.e., the nature of a spectrum depends on specific selection rules, so that the complexity of a spectrum is not as great as might be expected from first consideration of an energy level diagram. Wavelengths/Å for Intense X–ray • The fact that the wavelength of a line of given type decreases as the atomic number of the element increases is rather important in that it means that an X-ray from a given element must be able to cause inner shell ionization and, hence, emission of radiation of lower energy from any lighter element. Characteristics and Identification Of Wavelengths • Identification and measurement of concentration of elements based on the fact that primaryemission x-rays emitted by an element excited by an electron beam have a wavelength characteristic of that element and an intensity related to its concentration. It may be performed by an electron probe microanalyzer, an electron microscope microanalyzer, or by an electron microscope, or scanning electron microscope, fitted with an x-ray spectrometer. Electron Beam Sources In electron beam sources, X-rays are produced by heating a cathode to produce high-energy electrons; these electrons are energetic enough to ionize off the cathode and race towards a metal anode (the target) where, upon collision, X-rays are given off from the target material in response to the colliding electrons. The Duane-Hunt law The maximum photon energy corresponds to total stopping of the electron and is given by: hvo = (hc)/o = Ve vo is the maximum frequency V = accelerating voltage e = electron charge Continuum Spectra from Electron Beam Sources • In an X-ray tube, electrons produced at a heated cathode are accelerated toward a metal anode by a potential as great as 100kV; upon collision, part of the energy of the electron beam is converted into X-Rays. Under some conditions only a continuum spectrum is results. The continuum X-Ray spectrum is characterized by a welldefined, short wavelength limit, which is dependent upon the accelerating voltage but independent of the target material. The continuum radiation from an electron beam source results from collisions between the electrons of the beam and the atoms of the target material. Line Spectra from Electron Beam Sources • Bombardment of a molybdenum target produces intense emission lines. The emission behavior of molybdenum is typical of all elements having atomic numbers greater than 23, that is, the X-Ray line spectra are similar when compared with ultraviolet emission and consist of two series of lines. • Line spectra are composed of distinct lines of color, or in the case of our graphs, sharp peaks of large intensity at a particular wavelength. Line spectra are characteristic of elements and compounds when excited (energized) under certain conditions. These spectra helped develop the current atomic theories. Line spectra thus provide a “fingerprint” unique to each element, and as with continuous spectra, the combination of the prominent lines in the spectrum produce the observe light color. Line Spectra from Electron Beam Sources X-ray Fluorescence Since X-rays are rather energetic, excitation of sample electrons will give rise to fluorescence as the sample electrons are excited and return to their ground states in a series of electronic transitions. Bragg Equation sin = (n)/2d = angle of incidence = wavelength d = interplane distance of crystal Diffraction of X-rays by a crystal X-ray Monochromator and Detector References http://www.anachem.umu.se/jumpstation.ht m http://userwww.service.emory.edu/~kmurra y/mslist.html http://www.chemcenter/org http://www.sciencemag.org