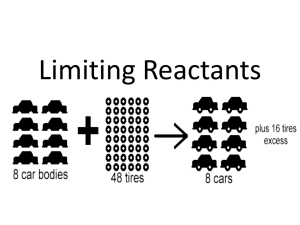
2 mole ratios 1112 cp2
advertisement
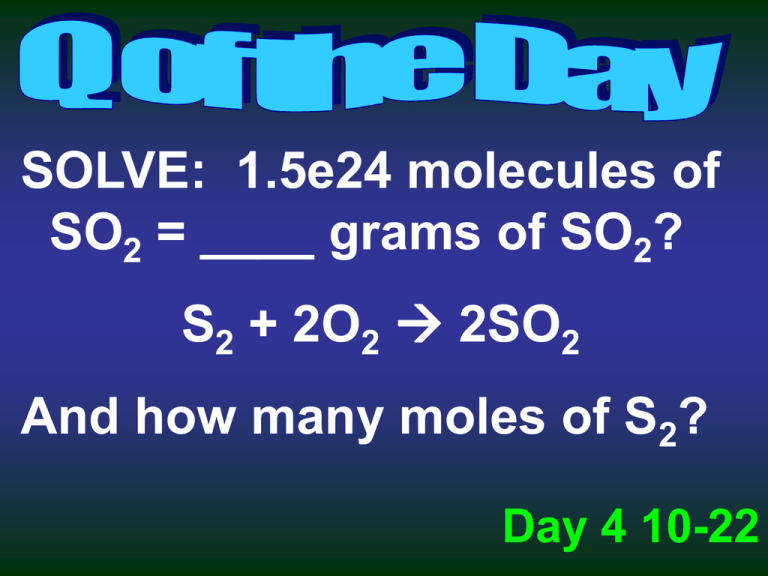
SOLVE: 1.5e24 molecules of SO2 = ____ grams of SO2? S2 + 2O2 2SO2 And how many moles of S2? Day 4 10-22 Limiting Reactants Limiting Reactant – the reactant that limits the amounts of the other reactants that can combine and the amount of product that can form in a chemical reaction – the one you will run out of Excess Reactant – any substance that is not used up completely in a reaction – left over Limiting Reactants To determine the Limiting Reactant – use either reactant to calculate the amounts necessary for reaction Example: 4NH3(g) + 6NO(g) 5N2(g) + 6H2O(l) Given the following, determine the limiting reactant: 40 g NH3 41 g NO Limiting Reactants Example: 4NH3(g) + 6NO(g) 5N2(g) + 6H2O(l) Given the following, determine the limiting reactant: 40 g NH3 41 g NO Limiting Reactants 2Na + Cl2 → 2NaCl Start with 4 moles of Na and 3 moles of Cl2: limiting = ? excess = ? Limiting Reactants 2Na + Cl2 → 2NaCl Start with 26 grams of Na and 35.5 grams of Cl2: limiting = ? excess = ? 1. If you start with 2.5 moles of solid aluminum and allow it to react with 2.5 moles of CuCl2 which reactant will be left over at the end? 2. Why is today Mole Day? Day 5 10-23 1. Which reactant determines your theoretical yield? Day 6 10-24 Percent Yield Percent Yield = Actual Yield X 100 Theoretical Yield the percent yield is a measure of the efficiency of a reaction carried out in the lab. - a perfect percent yield would be a 100% Example # 1: Calcium carbonate, which is found in seashells, is decomposed by heating. The reaction is below: CaCO3(s) CaO(s) + CO2(g) What is the theoretical yield of CaO if 24.8 grams of CaCO3 is heated? If only 12 grams of CaO are recovered, what is the percent yield? Percent Yield = Actual Yield X 100 Theoretical Yield What could be the cause? Quarterly Practice A solution containing 3.50 g sodium phosphate is mixed with a solution containing 6.40 g barium nitrate. After the reaction is complete, 4.85 g barium phosphate are collected. 1. What is the limiting reagent? 2. What is the theoretical yield of barium phosphate? 3. What is the percent yield of barium phosphate? 2Na3PO4(aq) + 3Ba(NO3)2(aq) 6NaNO3(aq) + Ba3(PO4)2(s) molar masses: Na3PO4 = 163.9 Ba(NO3)2 = 261.3 Ba3(PO4)2 = 601.9 12 Quarterly Practice A solution containing 3.50 g sodium phosphate is mixed with a solution containing 6.40 g barium nitrate. After the reaction is complete, 4.85 g barium phosphate are collected. 1. What is the limiting reagent? 2. What is the theoretical yield of barium phosphate? 3. What is the percent yield of barium phosphate? 2Na3PO4(aq) + 3Ba(NO3)2(aq) 6NaNO3(aq) + Ba3(PO4)2(s) If you start with 3.50 g Na3PO4 you NEED 8.37 g Ba(NO3)2 So Barium nitrate is limiting and you start your theoretical with 6.40 g barium nitrate Theoretical yield = 4.91 13 % yield = 98.8 % Assignment Review sections 12.1 and 12.2 and complete # 8 on page 389, # 11 on page 391, # 13 on page 393, and # 23 on page 398 – due tomorrow Get to # 11 Hand in assignment from Friday Assignment Review sections 12.1 and 12.2 and complete #s 5, 6, 8, 9, and 10 on page 389, #s 11 and 12 on page 391, and #s 13 and 14 on page 393 – due beginning of class Tuesday 10-29 Complete #s 21-25 on page 398 – due Wednesday 10-30