ChemistryName: Ms. Boon Period: ______ Date: Thermochemistry
advertisement

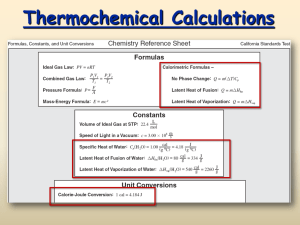
Chemistry Ms. Boon Name: _________________________________ Period: ______ Date: _______________ Thermochemistry Notes 3 Catalyst: Analyze the heating curve posted on the board here. Specific Heat Review: Use your notes to do the following specific heat problem. 3000 g of water are heated from 50 °C to 99°C. The specific heat of water is 4.18 J/g*K. How much heat was absorbed? Write the specific heat equation here: Set-up and Solve: Notes: Heat Transfer During Phase Changes We discussed the fact that temperature does not increase during a phase change. Instead, all the energy that is released or absorbed is used to power the phase change. On the phase change diagram this portion of the heating curve has a slope of zero. Today we will calculate how much heat is absorbed or released during a phase change. We will use water as our example, but a similar calculation can be done for other substances. The amount of heat absorbed or released during a phase change is generally called the latent heat of transformation. Different substances have different latent heats of transformation. Also, different phase changes absorb or release different amounts of heat. For example, less energy is absorbed to melt ice than is absorbed when water is vaporized. Table 1 contains the heats of transformation for water. Table 1: Heats of Transformation for H2O Analyzing Data. Use the table to answer the following questions. Change of State Energy Term Joules/gram 1. How do the heats of transformation for water compare to (J/g) the specific heat of water? Fusion (solid to Heat of Fusion 333.9 2. How many joules are released when 1 gram of water freezes? 3. Which requires more energy: melting or vaporization? 4. Why is the heat of fusion the same as the heat of crystallization? liquid) Freezing (liquid to solid) Vaporization (liquid to gas) Condensation (gas to liquid) Heat of Crystallization Heat of Vaporization Heat of Condensation 333.9 2256.8 2256.8 Latent Heat of Transformation Calculations If we know the latent heat of transformation of a substance, we can calculate the amount of heat energy absorbed or released during a phase change. The formula: Q = m × ΔHtranformation Chemistry Ms. Boon Name: _________________________________ Period: ______ Date: _______________ Example 1: How much heat is required to melt 1000 g of ice at 0°C? Phase Change Calculations Example 2: How much heat is needed to vaporize 200 g of water at 100°C? Example 3: How much ice, at 0°C, can be melted by 100 J of heat? (1) How much heat is released when 500 g of water freezes at 0°C? (2) How much heat is released when 2500 g of water condenses at 100°C? (3) How much water, at 100°C, can be vaporized by 1000 J of heat? Joule and Calorie Conversions: 1 calorie (cal) = 4.187 Joules (J) and 1 Joule (J) = 0.239 calories (cal) (4) What is the heat of fusion of water expressed in calories? (5) What is the specific heat of aluminum expressed in Cal/g*K? Molar Conversions: The Molar Heat of Transformation is the amount of heat absorbed or released during a phase change of 1 mole of a substance. (6) Calculate the molar heat of vaporization for water. (Step 1: Calculate the molar mass of water. Step 2: Multiply by the heat of transformation given in J/g) (7) Calculate the molar heat of fusion for water. Draw a phase change diagram for a substance with the following characteristics: (a) specific heat: 2.0 J/g*K, (b) Heat of fusion: 100 J/g, (c) Heat of vaporization: 300 J/g.