Chemical Kinetics
advertisement

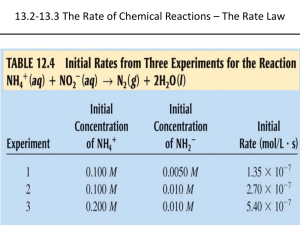
Chemical Kinetics How are chemical reaction rates determined and what factors affect these rates? Objectives To understand rates of reaction and the conditions affecting rates. To derive the rate equation, rate constant, and reaction order from experimental data. To use integrated rate laws. To understand collision theory of reaction rates and the role of activation energy. To relate reaction mechanisms and rate laws. Chemical Kinetics Ch 14 hmwk problems: 11 – 15, 20, 21, 25, 29, 33, 38, 45, 47, 50, 55, 58, 59, 62, 66, 69, 87, 98 Why are Kinetics Important? Medicinal: How quickly or slowly will a medication work? Environmental: Is the rate at which ozone depletion occurs equal to the rate at which it is formed? Industrial: How long will it take to produce a chemical? Is there a way to change this? What are Kinetics Anyway? Chemical Kinetics is the study of chemical reaction rates, or speeds. Can you think of an example of a chemical reaction with a fast rate? combustion of gasoline Can you think of an example of a chemical reaction with a slow rate? formation of a diamond What Makes a Reactions Occur? If a reaction is to occur between two or more reactants, the reactants must come in contact with each other, and have enough energy to both beak existing bonds and form new bonds. The rate at which these reactants come in contact with each other affects the rate of the overall chemical reaction. Factors Affecting Reaction Rates The Physical State of the Reactants: Reactions are limited by the area of contact between two reactants. Therefore, the greater the surface area of a solid reactant, the faster a reaction can proceed. Factors Affecting Reaction Rates The Concentration of Reactants: As the concentration of one or more reactants increases, the more frequently the reactants can collide, leading to increased reaction rates. Factors Affecting Reaction Rates The temperature at which the reaction occurs: The rate of a chemical reaction occurs increases with increasing temperature. As the molecules move more rapidly, they collide more frequently and with greater energy, increasing the reaction rate. Factors Affecting Reaction Rates The presence of a catalyst: Catalysts are reagents that increase the speed of a reaction without being consumed in the reaction by affecting how the reactants collide together. We will discuss this in greater depth later. Reaction Rates Since a rate is expressed as change over time, a reaction rate is expressed as the change in the concentration of reactants over time. For a simple reaction, A —› B, the rate would be can be described as the rate of appearance of B or the rate of disappearance of A, since one mole of A will produce to one mole of B. Reaction Rates For a simple reaction, A —› B, the rate would be expressed as either Δ[B]/ Δt or -Δ[A]/ Δt Rates are always expressed as positive quantities, therefore, a negative sign must be used because the [A] decreases as the reaction progresses A reaction vessel initially contains 1.00 M of reactant A. Twenty seconds later, the reaction vessel contains only 0.54 M of reactant A and 0.46 M of product B. Calculate the average rate of the disappearance of reactant A. Recall, A —› B, and rate = -Δ[A]/ Δt . Rate = -[0.54M – 1.00M]/(20s – 0s) Rate = 0.46M/20s Rate = 2.3 x 10-2 M/s Reaction Rates and Stoichiometry What if the reaction stoichiometry is more complicated than our simple 1:1 ratio? Let’s look at 2HI —› H2 + I2 Rate = -½ Δ[HI]/Δt = Δ[H2]/ Δt = [I2]/ Δt Reaction Rates and Stoichiometry In general, for the reaction aA + bB —› cC + dD the rate is given by Rate= -1/aΔ[A]/Δt = -1/bΔ[B]/Δt = 1/cΔ[C]/ Δt = 1/dΔ[D]/ Δt How is the rate at which ozone disappears related to the rate at which oxygen appears in the reaction 2O3 —› 3O2? Rate = -1/2Δ[O3]/Δt = 1/3Δ[O2]/Δt If the rate at which O2 appears is 6.0 x 10-5 M/s at a particular instant, at what rate is O3 disappearing at the same time? Rate = -1/2Δ[O3]/Δt = 1/3Δ[O2]/Δt Rate = -1/2Δ[O3]/Δt = 1/3(6.0 x 10-5 M/s) Rate = -Δ[O3]/Δt = 2/3(6.0 x 10-5 M/s) Rate = -Δ[O3]/Δt = 4.0 x 10-5 M/s You should now be able to complete # 11 & 12 Rate Laws The rate law is an equation that relates the rate of a reaction to the concentration of reactants (and catalysts) raised to various powers The rate law for our last example, aA + bB —› cC + dD, would be expressed as Rate = k[A]m[B]n, where k is the rate constant and m and n are typically small, whole numbers. Rate Laws k in the rate law is the rate constant defined as a proportionality constant in the relationship between rate and concentrations. The rate constant is fixed at a particular temperature (but will change as the temperature changes) Rate Laws We can classify reactions by their orders – The reaction order, with respect to a given reactant species, equals the exponent of the concentration of that species in the rate law, as determined experimentally – The overall order of the reaction equals the sum of the orders of all reactant species in the rate law – In the case Rate = k[A]m[B]n, the overall order of this reaction is given by m+n. Rate Laws For the following reaction, the rate law can be written as is indicated below: NH4+ + NO2- —› N2 + 2H2O Rate = k[NH4+][NO2-] Because the exponent of [NH4+] is one, the rate in NH4+ is first order. The rate in [NO2-] is also first order. The overall reaction order is second order. The exponents in a rate law indicate how the rate is affected by the concentration of each reactant. For the following reaction, the rate law can be written as is indicated below: NH4+ + NO2- —› N2 + 2H2O Rate = k[NH4+][NO2-], What is the effect on the rate if [NO2-] doubles? Because the exponent of [NO2-] in the rate law is one, doubling its concentration will double the rate. What is the effect on the rate if [NH4+] triples? Because the exponent of [NH4+] in the rate law is one, tripling its concentration will triple the rate. For a given reaction, the rate law is given by Rate = k[NO2]2 What is the order of the reaction with respect to NO2? Because the exponent of NO2 in the equation is 2, the reaction is second order with respect to NO2. Because the concentration of only one reactant is expressed in the rate law, the overall order is also second. What is the effect on the rate if [NO2] triples? If the concentration triples, then the rate increases by 9 since [3]2. Rate Laws: Additional Examples 2N2O5 —› 4NO2 + O2 CHCl3 + Cl2 —› CCl4 + HCl H2 + I2 —› 2HI Rate = k[N2O5] Rate = k[CHCl3][Cl2]½ Rate = k[H2][I2] Note: While exponents in rate laws are often the same as the coefficients in a balanced equation, this is not a rule! Exponents in a rate law can only be determined experimentally! Determining a Rate Law Experimentally – Initial Rate Method Trial [NO] [H2] R0 (M/s) 1 0.10 0.10 1.23 x 10-3 2 0.10 0.20 2.46 x 10-3 3 0.20 0.10 4.92 x 10-3 Using the data above, determine the rate law, calculate the value of k and determine the rate when [NO] = 0.050M and [H2] = 0.150 M. Determining a Rate Law Experimentally – Initial Rate Method A. Determine the rate law Recall, the Rate = k[NO]x[H2]y. We can determine the values of x and y by using the experimental data and the equation and solving for the unknown variables. Determining a Rate Law Experimentally – Initial Rate Method Let’s compare trial two to trial one: Rate 2 = [NO]x[H2]y = [0.10]x[.20]y Rate 1 = [NO]x[H2]y = [0.10]x[.10]y We can simplify our expression and substitute the values for Rate 1 and Rate 2 Rate 2 = 2.46 x 10-3 = [.20]y Rate 1 = 1.23 x 10-3 = [.10]y Notice, we are only left with one unknown variable to solve for. Determining a Rate Law Experimentally – Initial Rate Method Rate 2 = 2.46 x 10-3 = [.20]y Rate 1 = 1.23 x 10-3 = [.10]y 2y = 2 y must equal 1. So far we have determined that Rate = k[NO]x[H2]1. We must repeat the process to solve for x by comparing trial 3 to trial 1. Determining a Rate Law Experimentally – Initial Rate Method Rate 3 = [NO]x[H2]y = [0.20]x[.10]y Rate 1 = [NO]x[H2]y = [0.10]x[.10]y Rate 3 = 4.92 x 10-3 = [.20]x Rate 1 = 1.23 x 10-3 = [.10]x 2x = 4 X must equal 2. Therefore, the experimentally determined rate law is Rate = k[NO]2[H2]1 Determining a Rate Law Experimentally – Initial Rate Method B. Calculate the value of k If Rate = k[NO]2[H2]1, then simple substitution from the data table will allow us to calculate k. Rate = k[NO]2[H2]1 (use trial 1) 1.23 x 10-3M/s = k[0.10M]2[0.10M]1 1.23 x 10-3M/s = k(0.0010M3) 1.2 M-2·s-1 = k Determining a Rate Law Experimentally – Initial Rate Method C. Determine the rate when [NO] = 0.050M and [H2] = 0.150 M Since Rate = 1.2M-2·s-1[NO]2[H2]1 Rate = 1.2M-2·s-1[0.050M]2[0.150M]1 Rate = 4.5 x 10-4 M/s You should now be able to complete # 13, 14, 15, 20, 21, 25 Instantaneous Rates Calculated from a graph of experimental data that represents concentration vs. time. Instantaneous Rates Recall, the slope of a graph is Δy/Δx. In a concentration vs. time graph, the Δy = Δ[reactant] and Δx = Δtime. Therefore, the slope of the graph = Δ[reactant]/ Δtime and will yield the instantaneous rate of reaction progression at any given point in time. Average reaction rates can be determined from the average of several calculated instantaneous rates. Rate Orders Recall, the overall order of a reaction is equal to the sum of the exponents in a rate law Rate = k[A]0 or Rate = k is zero order Rate = k[A]1 is first order Rate = k[A]2 or Rate = k[A][B] is second order Rate = k[A]3 or Rate = k[A]2[B] is third order A closer look at Zeroorder Reactions Rate = k[A]0 or Rate = k is zero order This is referred to as the differential rate law. If we integrate the above equation, we find a very useful equation: [A] = -kt + [A0] This will allow us to solve for unknown concentration values of zero-order reactions A closer look at Zeroorder Reactions A zero-order reaction begins with [X] = 0.50 M. How long does it take to react all but 0.10M if k=0.0410 M/min? What is the half-life? What is the [X] after 5 min? A closer look at Zeroorder Reactions A. [X] = 0.10 M, [X0] = 0.50 M and k=0.0410 M/min [A] = -kt + [A0] [0.10M] = -(0.0410M/min)t + [0.50M] -0.40M = -(0.0410M/min)t 10. min = t A closer look at Zeroorder Reactions B. What is the half-life? Recall, a half-life is the time it takes for half a reactant to be consumed. So, if [X0] = 0.50 M, then [X] must equal 0.25 M If [A] = -kt + [A0], then [0.25M] = -(0.0410M/min)t½ + [0.50M] -0.25M = -(0.0410M/min)t½ 6.1 min = t ½ A closer look at Zeroorder Reactions C. What is the [X] after 5 min? If [A] = -kt + [A0], then [X] = -(0.0410M/min)5min + [0.50M] [X] = -.205M + 0.50M [X] = 0.30 M A closer look at Zeroorder Reactions Notice, [A] = -kt + [A0] closely mimics y = mx + b Therefore, a graph of change in concentration vs. time will be linear for a zero-order reaction. A closer look at Firstorder Reactions Rate = k[A]1 is first order This is referred to as the differential rate law. If we integrate the above equation, we find a the integrated rate law for first order equations: ln[At] - ln[A0] = -kt Or ln[At] = -kt + ln[A0] (y) = (mx) + b This will allow us to solve for unknown concentration values of first-order reactions A closer look at Secondorder Reactions Rate = k[A]2 or is second order This is referred to as the differential rate law. If we integrate the above equation, we find a the integrated rate law for second order equations: 1/[At] = kt + 1/[A0] (y) = (mx) + (b) This will allow us to solve for unknown concentration values of second-order reactions Determining Order From Graphical Data Time (min) 0 200 400 600 800 1000 1200 1600 [X] 0.0200 0.0160 0.0131 0.0106 0.0086 0.0069 0.0056 0.0037 An experiment was conducted were a reaction was allowed to proceed and the reactant concentration was measured over a period of time. What is the order of this reaction? Determining Order From Graphical Data Recall, the integrated rate law of zero, first and second order reactions can be rearranged in the form of y=mx + b Zero-order: [A] = -kt + [A0] First-order: ln[At] = -kt + ln[A0] Second-order: 1/[At] = kt + 1/[A0] If we create three plots of our data, we can determine order: [X] vs. t; ln[X] vs. t; 1/[X] vs. t. The plot that yield a linear graph will determine its order. Determining Order From Graphical Data Notice, only one graph yields a straight line: The plot of ln[X] vs. t. Therefore, the reaction must be first order. You should now be able to complete # 29, 33, 38 A Microscopic View of Reaction Rates Consider the reaction NO + O3 —› NO2 + O2 where Rate = k[NO][O3] Imagine these reactants in rapid, random motion inside a flask. They strike the walls of the reaction vessel and collide with other molecules. Will all collisions result in a chemical reaction? What conditions must be met for these reactants to react together? How a Chemical Reaction Takes Place Only a tiny fraction of reactant collisions leads to reaction. Why? – Reactant molecules must collide in the proper orientation. – They must collide with the minimum energy required to initiate a chemical reaction. This is called activation energy (Ea) and the value varies from reaction to reaction. Activation Energy The diagrams shows the change in potential energy of the molecules during the reaction. Energy must be supplied to the reactants to stretch and break reactant bonds into an intermediate form (represented at the peak of the graph) before proceeding to the final product. The energy difference between the starting molecule and the highest energy along the path represent the activation energy. The difference in the potential energies of the reactants and products indicate whether the reaction was endo- or exothermic. The Arrhenius Equation Most reaction rate data is based on three factors 1. The fraction of molecules possessing the minimum Ea or greater 2. The number of collisions occurring per second 3. The fraction of collisions that have the appropriate orientation Arrhenius related these factors mathematically: k = Ae-Ea/RT The frequency factor, A, is considered constant. The Arrhenius Equation The Arrhenius can be written in several forms: k = Ae-Ea/RT lnk = -Ea/RT + lnA ln(k1/k2) = Ea/R (1/T2 – 1/T1) (notice this last formula closely mimics y=mx + b) Using the Arrhenius Equation Use the data to determine the activation energy for the zero-order decomposition of HI on a platinum surface. Temp (K) Rate Constant (M/s) 573 2.91 x 10-6 673 8.38 x 10-4 773 7.65 x 10-2 We can determine the activation energy for a reaction from a plot of the lnk vs. 1/T. According to ln(k1/k2) = Ea/R (1/T2 – 1/T1), the slope of this graph will equal Ea/R. Using the Arrhenius Equation Temp (K) Rate Constant (M/s) lnk 1/T (K-1) 573 2.91 x 10-6 -12.75 0.00175 673 8.38 x 10-4 -7.08 0.00149 773 7.65 x 10-2 -2.57 0.00129 ln k vs. 1/T 0 0 0.0005 0.001 0.0015 -2 ln k -4 -6 y = -22115x + 25.926 -8 -10 -12 -14 1/T (K-1) 0.002 Using the Arrhenius Equation The graph yields a straight line with a slope of -2.2x104K. According to the equation, ln(k1/k2) = Ea/R (1/T2 – 1/T1), this equals Ea/R. So, -2.2x104 K= Ea/R -2.2x104 K= Ea/8.314 J/K·mol Ea= 1.8 x 102 kJ/mol You should now be able to complete # 45, 47, 50 Reaction Mechanisms We know that reactants must change form (often forming an intermediate state) in order to proceed to becoming products. This process can be described in much greater detail if we look at a chemical reaction as occurring over a series of steps. This is referred to as a reaction mechanism. Reaction Mechanisms Reaction mechanisms can be very simple and involve only one step, or very complicated, involving many steps. Reaction mechanisms are often speculated. It is very difficult to scientifically prove that a chemical reaction happens in a specific way. Reaction Mechanisms The steps of a reaction mechanism can be described by the number of molecules that participate as reactants in the step. This is called the molecularity of the reaction. H3CNC —> H3CCN unimolecular NO + O2 —> NO2 + O2 bimolecular And so on Reaction Mechanisms Reactions involving only one step are referred to as elementary reactions Reaction involving several steps are referred to as multistep reactions. This often involves the formation and consumption of an intermediate. Multistep Mechanisms Let’s consider the reaction NO2 + CO —> NO + CO2 Studies of the reaction have shown that it most likely occurs in two steps, NO2 + NO2 —> NO3 + NO NO3 + CO —> NO2 + CO2 Thus, we say the reaction occurs by a two-step mechanism. Each step is represented by an elementary reaction and the rate law can be written directly from its molecularity. Multistep Mechanisms The chemical equations for the elementary reactions in a multistep mechanism must always add to give the chemical equation of the overall process. NO2 + NO2 —> NO3 + NO NO3 + CO —> NO2 + CO2 2 NO2 + NO3 + CO —> NO2 + NO3 + NO + CO2 This equation can be simplified by eliminating substances that appear on both sides to yield: NO2 + CO —> NO + CO2 Notice, NO3 appears in the reaction mechanism, but it neither a reactant nor a product. It is formed in one elementary step and consumed in the next. NO3 is an intermediate. Multistep Mechanisms Each step of a mechanism has its own rate law. Each step is represented by an elementary reaction and the rate law can be written directly from its molecularity. Step 1: NO2 + NO2 —> NO3 + NO Rate1 = k1[NO2]2 Step 2: NO3 + CO —> NO2 + CO2 Rate2 = k2[NO3][CO] It is important to remember that the overall rate law of a chemical reaction does not necessarily follow the molecularity of the balanced chemical equation! It must be determined experimentally. Multistep Mechanisms Most chemical reactions occur by mechanisms involving two or more steps. These steps often occur at different rates. The overall rate of a chemical reaction is determined by the slowest step of its mechanism. This is referred to as the rate-determining step. Multistep Mechanisms – Slow Initial Step Let’s take another look at our example. Step 1: NO2 + NO2 —> NO3 + NO Step 2: NO3 + CO —> NO2 + CO2 Overall: NO2 + CO —> NO + CO2 If we know that step 1 is much, much slower than step two, step 1 must be ratedetermining. Therefore, the overall rate law for this reaction must be Rate = k[NO2]2 Multistep Mechanisms – Fast Initial Step If the initial step is fast, and therefore not rate-determining, it is likely that the ratedetermining step involves an intermediate as a reactant. Let’s look at another example: Step 1: NO + Br2 <—> NOBr2 (fast) Step 2: NOBr2 + NO —> 2NOBr (slow) Overall: 2NO + Br2 —> 2NOBr How would you write a rate law for the overall reaction? Multistep Mechanisms – Fast Initial Step You may have been tempted to say Rate = k[NOBr2][NO] However, notice that an intermediate appears in the rate law. The concentrations of intermediates are usually unknown. Thus, our rate law depends on an unknown concentration. We must rewrite our rate law to include only known quantities. But how??? Multistep Mechanisms – Fast Initial Step Let’s go back to the mechanism Step 1: NO + Br2 <—> NOBr2 (fast) Step 2: NOBr2 + NO —> 2NOBr (slow) Overall: 2NO + Br2 —> 2NOBr Write a rate law the first step Step 1: NO + Br2 <—> NOBr2 (fast) Rate1 = k1[NO][Br2] But notice the equilibrium? The rate above is for the forward reaction, what about the reverse? Rate-1 = k-1[NOBr2] Multistep Mechanisms – Fast Initial Step Write a rate law the second step Step 2: NOBr2 + NO —> 2NOBr (slow) Rate2 = k2[NOBr2][NO] Recall, the original overall rate law was written as Rate = k[NOBr2][NO]. We must rewrite the rate law without [NOBr2]. The three elementary rate laws written from the mechanism should give us plenty of substitution to choose from. Multistep Mechanisms – Fast Initial Step We must rewrite Rate = k[NOBr2][NO] using the elementary rate laws shown below. Rate1 = k1[NO][Br2] Rate-1 = k-1[NOBr2] Rate2 = k2[NOBr2][NO] Recall that because of equilibrium, Rate1 = Rate-1. So, k1[NO][Br2] = k-1[NOBr2] Notice, we can now solve for NOBr2. (k1/k-1) [NO][Br2] = [NOBr2] Multistep Mechanisms – Fast Initial Step Let’s make the substitution. Rate = k[NOBr2][NO] Rate = k(k1/k-1) [NO][Br2][NO] Since k’s are constant, let’s rename k(k1/k-1) as K. Rate = K [NO][Br2][NO] And now simplify Rate = K [NO]2[Br2] where K = k(k1/k-1). This is a viable rate law since the concentrations of all species in the rate law are known. You should now be able to complete # 55, 58, 59, 62 Catalysis A catalyst is a substance that changes the speed of a chemical reaction without undergoing a permanent chemical change. A catalyst can be homogeneous (in the same phase as the reacting molecules) or heterogeneous (in a different phase as the reacting molecules) Enzymes in the human body are examples of organic catalysts. Homogeneous Catalysis H2O2(aq) —> H2O(l) + O2(g) The decomposition of hydrogen peroxide can be catalyzed by I- (given by the dissociation of KI) as shown. Step 1: 2I-(aq) + 2K+(aq) + H2O2(aq) —> I2(aq) + 2H2O(g) Step 2: I2(aq) + H2O2(aq) —> 2I-(aq) + 2K+(aq) + O2(g) Notice, adding steps 1 & 2 yields 2H2O2(aq) —> 2H2O(l) + O2(g) How does this work? Homogeneous Catalysis As a general rule, a catalyst lowers the overall activation energy for a chemical reaction Heterogeneous Catalysis The initial set usually begins with adsorption of reactants onto the catalyst surface. Atoms/Ions on the surface of a solid are very reactive because of the unused bonding capacity. Be sure to read this section of your text book carefully so you understand how heterogeneous catalysis works!!! SAMPLE INTEGRATIVE EXERCISE Putting Concepts Together Formic acid (HCOOH) decomposes in the gas phase at elevated temperatures as follows: The decomposition reaction is determined to be first order. A graph of the partial pressure of HCOOH versus time for decomposition at 838 K is shown as the red curve in Figure 14.28. When a small amount of solid ZnO is added to the reaction chamber, the partial pressure of acid versus time varies as shown by the blue curve in Figure 14.28. Figure 14.28 Variation in pressure of HCOOH(g) as a function of time at 838 K. The red line corresponds to decomposition when only gaseous HCOOH is present. The blue line corresponds to decomposition in the presence of added ZnO(s). SAMPLE INTEGRATIVE EXERCISE continued (a) Estimate the half-life and first-order rate constant for formic acid decomposition. (b) What can you conclude from the effect of added ZnO on the decomposition of formic acid? (c) The progress of the reaction was followed by measuring the partial pressure of formic acid vapor at selected times. Suppose that, instead, we had plotted the concentration of formic acid in units of mol/L. What effect would this have had on the calculated value of k? (d) The pressure of formic acid vapor at the start of the reaction is 3.00 10 2 torr. Assuming constant temperature and ideal-gas behavior, what is the pressure in the system at the end of the reaction? If the volume of the reaction chamber is 436 cm3, how many moles of gas occupy the reaction chamber at the end of the reaction? (e) The standard heat of formation of formic acid vapor is ΔHf° = 378.6 kJ/mol. Calculate ΔHº for the overall reaction. Assuming that the activation energy (Ea) for the reaction is 184 kJ/mol, sketch an approximate energy profile for the reaction, and label Ea, ΔHº, and the transition state. You should now be able to complete # 66, 87, 88, 98 You Should Now Be Able To… Identify factors which affect reaction rates. Calculate the rate of production of a product or consumption of a reactant using mole ratios and the given rate. Determine the rate law for a reaction from given data, overall order and value of the rate constant (including units!) Determine the instantaneous rate of a reaction. You Should Now Be Able To… Use integrated rate laws to determine concentrations at a certain time, t, and create graphs to determine the order of a reaction. Also, determine the half-life reaction. Determine the activation energy for the reaction using the Arrhenius equation. Graphically determine the activation energy using the Arrhenius equation. Write the rate law from a given mechanism and identify catalysts and intermediates present.