Nuclear Binding Energy
advertisement
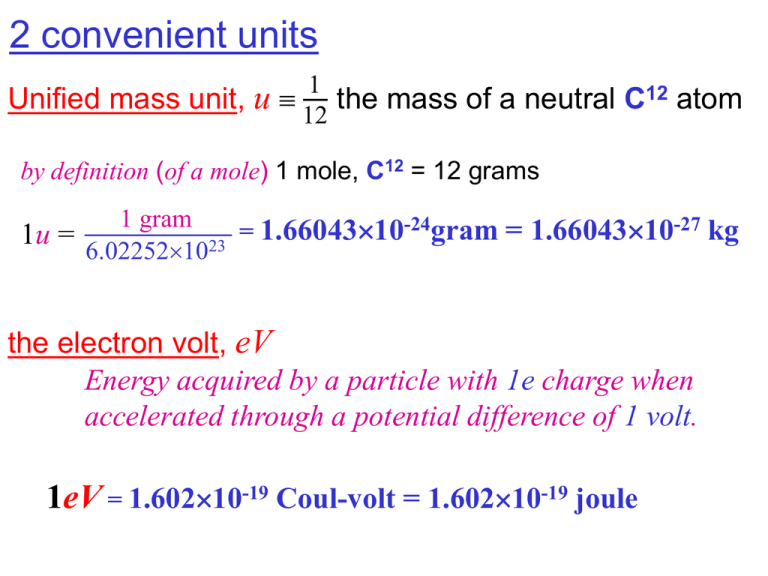
2 convenient units Unified mass unit, u 1 12 the mass of a neutral C12 atom by definition (of a mole) 1 mole, C12 = 12 grams 1 gram -24gram = 1.6604310-27 kg = 1.6604310 1u = 6.022521023 the electron volt, eV Energy acquired by a particle with 1e charge when accelerated through a potential difference of 1 volt. 1eV = 1.60210-19 Coul-volt = 1.60210-19 joule 1eV = 1.60210-19 Coul-volt = 1.60210-19 joule 1u c2 = 1.6604310-27 c2 kg 1.60210-19 joule/eV 8 m/sec) 2 (2.99810 = 1.6604310-27 kg 1.60210-19 joule/eV 1u c 2 = 9.315825108 eV = 931.58 MeV 1u = 931.58 MeV/c 2 Rest mass energy can be measure in terms of eV or MeV. Sometime mass is measure in the convenient unit of MeV/c2. 1 keV = 103 eV 1 MeV = 106 eV 1 GeV = 109 eV 1 TeV = 1012 eV • 1012 • 109 • 106 • 103 tera giga mega kilo (trillion) (billion, “80 Gigabyte hard drive”) (million, “128 Megabytes of RAM”) (thousand, 1 kilogram = 2.2 pounds) • 10– 2 • 10– 3 • 10– 6 • 10– 9 • 10– 12 • 10– 15 centi milli micro nano pico femto (hundredth, 1 in. = 2.54 cm) (thousandth, “milliVolt”) (millionth, micron=micrometer=10-6m) (billionth, “nanosecond”) (trillionth) With the nuclear volume the number of nucleons A the nuclear force is not sufficient to contract the nucleus to higher densities at its center. the nuclear force saturates the density of nucleons in the nucleus ~ constant Liquid Drop Model 1935-36 Bohr & von Weizsäcker Cross Section measurements help fix the effective range of the nuclear force Precision mass measurements reveal binding energies that tell us even more about the strength of the nuclear force Particle symbol rest energy in MeV or mass in MeV/c2 electron e muon 105.658357 neutral pion 0 134.9766 charged pion 139.57018 proton p 938.27200 neutron n 939.56533 deuteron H2 1875.580 triton H3 2808.873 alpha (He4) 3727.315 0.5109989 Know from relativity E and m intimately related Rest mass energy: E =moc2 For a free particle, total energy: E with p2 E2 or – = T + moc2 = moc2 = Eo = 2m2v2 = 2 Eo2v2/c4 then p 2c 2 = 2 2E 2 o E2 = p2c2 + Eo2 v ( 1 2 ) c 2 v 2 1 / 1 2 c and so: T KE = moc2 ( - 1) = c2 Dm Work done by an unbalanced force increases an object’s energy from a rest mass of moc2 moc2 or increases it’s mass from mo mo If balanced or resisted by a restoring force: increases an object’s POTENTIAL ENERGY Proton billiards: consider the head-on collision between 2 protons: m0 m0 Einitial m0c m0c 2 ' ( 2m0 )c 2 E final m0c m0c 2 ' ( 2m0 )c (1 )m0c 2 2 2 2 ' (1 ) / 2 revealing the intermediate state of “bound” protons to have a mass of ' ( 2m0 ) (1 )m0 > 2m0 !!! or consider the same collision in the center of momentum frame: E=m0c2 E=m0c2 Einitial m0c m0c 2 2 so here can’t have E=2m0c2 Einitial m0c m0c 2 2 E 2m0c 2m0c 2 2 The intermediate system of protons has more mass than two protons! For a repulsive potential combined rest mass > sum of the individual rest masses For an attractive potential combined rest mass < sum of the individual rest masses Rest energies of a proton + neutron = 938.27200+939.56533 = 1877.83733 MeV while of a deuteron H2 = 1875.61269 MeV 2.22464 MeV A deuteron cannot spontaneously decay into a proton and a neutron. To break up a deuteron 2.22464 MeV must be added by bombarding deuterons with an energetic beam of particles or electromagnetic radiation. Neutrons from a reactor striking protons are sometimes “captured” through the reaction n+pd+ For the alpha particle Dm= 0.0304 u which gives 28.3 MeV binding energy! For “bound states” the binding energy, B>0 representing the energy required to disintegrate the nucleus (into individual neutrons and protons) B Zm ( A Z ) m M ( A , Z ) p n nucleus 2 c B Z ( m m ) ( A Z ) m M ( A , Z ) p e n neutral atom 2 c B Zm ( A Z ) m M ( A , Z ) H n neutral atom 2 c Working in atomic mass units, u the difference between atomic rest mass energy M(A,Z) and atomic mass number A u is called the MASS DEFECT (or “mass excess”) D M ( A, Z ) Au Notice with Au Zmp + (A-Z)mn this is essentially the same as the Binding Energy D follows the conventions of mass spectroscopy measurements Peaks at ~8.795 MeV near A=60 Binding energy per nuclear particle (nucleon) in MeV for A>50 ~constant 8-9 MeV Mass Number, A This confirms the short range ascribed to the nuclear force… it must involve only nearest neighbor nuclei If the binding involved contributions from all nucleons the total number of pair-wise bonds A( A 1) A2 then B A A (not constant!) Nuclear Binding Energy Curve The binding energy curve is obtained by dividing the total nuclear binding energy by the number of nucleons. The fact peak in the binding energy curve near iron means that either the breakup of heavier nuclei (fission) or the combining of lighter nuclei (fusion) will yield nuclei which are more tightly bound (less mass per nucleon). The binding energies of nucleons are in the range of millions of electron volts compared to tens of eV for atomic electrons. Whereas an atomic transition might emit a photon in the range of a few electron volts, perhaps in the visible light region, nuclear transitions can emit gamma-rays with quantum energies in the MeV range. The iron limit: The buildup of heavy elements by the nuclear fusion processes in stars is limited to elements below iron, since the fusion of iron would subtract energy rather than provide it. Iron-56 is abundant in stellar processes, and with a binding energy per nucleon of 8.8 MeV, it is the third most tightly bound of the nuclides. Its average binding energy per nucleon is exceeded only by 58Fe and 62Ni, the nickel isotope being the most tightly bound of the nuclides. The Most Tightly Bound Nuclei 62Ni M. P. Fewell, American Journal of Physics. (56Fe actually comes in a close third) B/A (keV/A) 62Ni 8794.60 +/- 0.03 58Fe 8792.23 +/- 0.03 56Fe 8790.36 +/- 0.03 60Ni 8780.79 +/- 0.03 The most tightly bound nuclides are all even-even nuclei. The high binding energy of the “iron group” of around A=60 is significant in the understanding of the synthesis of heavy elements in the stars. The Semi-emperical Mass Formula is an approximate fit to all this data To 0th order: M ( A, Z ) Zm p ( A Z )mn The 1st correction to this (the “mass defect” or binding energy) ~proportional to volume B/A (MeV) EV av A true enough for very small A ! A But the nuclei at the surface have fewer nearest-neighbor bonds! So we’ve obviously over-estimated! by something the nucleus’ surface area Es a s A 2/3 recall r A1 / 3 With these effects alone, all isobars might be expected to be stable though clearly we’ve seen a narrow band of stability in the N vs A plane for light nuclides: N=Z (A = 2Z) for heavy nuclides: A>2Z There’s a repulsive Coulomb energy building up with all those protons! Coulomb Energy The work done in collecting and compacting total charge Q into a sphere of volume 4 R 3. 3 potential at the surface of a spherical concentration of charge kq R with charge density: Q ( 4 / 3)r 3 so at r: 3 2 k ( 4r 3 / 3) Q 4 r / 3 r kQ V k r R3 4R 3 / 3 r Bringing an additional dq=4r2dr to the surface at r requires: kQ 2 kQ 4 5 2 Vdq 3 r 4r dr r dr 3 R R Coulomb Energy kQ4 Q 1 5 kQ 4 R 5 R Integrating: 0 r dr 3 3 3 R 4R / 3 5 R 1 3Q 2 1 4 0 5 R Motivating us to add a correction term for this Coulomb energy e2 Z(Z-1)e2 Q2 Z ( Z 1) R number of pairings Ec ac Z ( Z 1) A1 / 3 RA1/3 B av A as A ac Z ( Z 1) A 1 / 3 2/3 volume term grows with addition of each interacting nuclei surface term corrects for nuclei at the surface (not completely surrounded by nearest neighbors) Coulomb term accounts for (repulsive) energy built up in the accumulation of protons Mass defect D (MeV) A = 127 isobars -80 -80 -84 -84 -86 -86 -88 -88 50 52 54 56 Atomic number, Z Think of the stability band as a contour map of a valley: A consequence of the exclusion principal: less economical (more expensive in energy) to have more of one type of nucleon than the other: (A/ 2 Z) a symmetry sym A 2 Like the “closed shell” electronic configurations of the noble gases we see “magic numbers” recurring…marking tightly bound, extraordinarily stable nuclear configurations. =4He (with Z=N=2) and 16O (Z=N=8) are doubly magic! including to a lesser extent, marked stability for nuclei with paired protons and/or paired neutrons (like the closed energy levels of atomic orbitals where electrons with opposite spins cancel) Since nuclei with paired protons or paired neutrons tend to be more tightly bound, we introduce the pairing energy term: ap A3 / 4 ( A) 0 ap A3 / 4 even Z, N Z=N=0 odd Z, N volume term grows as nuclei added surface term corrects for surface nuclei B av A a s A 2/3 ac Z ( Z 1) A (A/ 2 Z) asym A symmetry term drives number of protons ≈ neutrons Coulomb term repulsion due to protons 1 / 3 2 ( A) pairing energy term increased stability for paired nuclei M ( A, Z ) Zm p ( A Z )mn B( A, Z )c 2 B( A, Z ) av A as A 2/3 1 / 3 ac Z ( Z 1) A asym ( A / 2 Z ) / A ( A, Z ) 2 av=15.5 MeV ac=0.72 MeV ap=34.0 MeV as=16.8 MeV asym=23.0 MeV Atomic shell model Nuclear shell model each nucleon, separately occupies its own energy level, with each nucleon-type (p,n) filling energy levels separately Each energy level, n exists with an angular quantum number: s p d f g … ℓ = 0, 1, 2, 3, 4, … each with a different angular wave function Yℓ,m(,) Because of spin (2 possible orientations of each nucleon) any energy level n,ℓ accommodates 2ℓ+1 protons 2ℓ+1 neutrons s: ℓ = 0 p: ℓ = 1 d: ℓ = 2 f: ℓ = 3 These are different particles – They don’t mutually exclude each other! coupled with spin: s1/2 p1/2 p3/2 d3/2 d5/2 f 5/2 f 7/2 J=0+½ The total (magnitude) of J is limited to -|ℓ-s|…|ℓ+s| in unit steps! There is a 1f5/2 energy level that can hold 5 2( ) ???? 1 6 protons with different mj values 2 and another that can hold 6 neutrons. Consider: 27 13 Al (ground state) 2 4 2 Protons: (1s1/2) (1p3/2) (1p1/2) (1d5/2) 2 4 2 5 Neutrons: (1s1/2) (1p3/2) (1p1/2) (1d5/2) 6 1 un-paired proton with j=5/2 so aluminum: I=5/2 Consider: 9 Be (ground state) 2 2 2 3 p: (1s1/2) (1p3/2) (1p1/2) (1d5/2) n: (1s1/2) (1p3/2) (1p1/2) (1d5/2) 1 un-paired neutron with j=3/2 so: I=3/2 Consider: 9 B (ground state) 2 3 2 2 p: (1s1/2) (1p3/2) (1p1/2) (1d5/2) n: (1s1/2) (1p3/2) (1p1/2) (1d5/2) 1 un-paired proton with j=3/2 so: I=3/2 Magnetic Dipole Moment B classically energy Emag = -B = 1 c current area For a point particle q, velocity v, circular orbital radius r v I q 2r frequency! 1 = c current area q 1 qvr v 2 ( mvr ) = q r = c 2mc 2 2r L q L but maybe too classical! 2mc We still expect J quantum mechanically and write e g measures the g J (expected) 2mc deviation from 1 = c quantum mechanically J is quantized! J j e g j 2mc g 0 j 0 = “magneton” For atomic physics (involving electrons) “Bohr magneton” e 14 B 0.5788 10 MeV / G 2me c 5 5.7885 10 eV / T For nuclear physics “nuclear magneton” e N 3.1525 10 18 MeV / G 2m p c 3.15255 10 8 eV / T For the neutron: g= 3.826083 0.0000018 0 !









