Extension.Station
advertisement

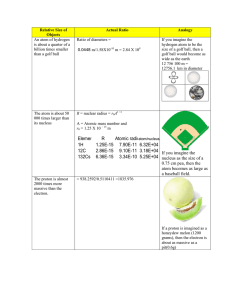
CHALLENGE ACTIVITIES 1. The activities presented in this class period highlighted the contributions of several historically important individuals, but many more scientists and theorists have contributed to the development of contemporary atomic theory. To expand on the historical development of atomic theory, research the contributions of one or more of the following individuals. After conducting your research, write a brief essay (2-3 paragraphs) that details (1) what the scientist discovered, (2) the experiment that the scientist used to make that discovery, and (3) any other relevant information about that scientist. Feel free to supplement your writing with diagrams, drawings, and/or graphs. Topic Contributor(s) Atomism Leucippus Nucleus of the atom Hans Geiger, Ernest Marsden Discovery of neutrons Irene Joliot-Curie, James Chadwick Isotopes F.W. Aston Atomic number Henry Moseley Electrons as particles and waves Louis de Broglie The nature of light Christian Huygens (1629–1695), Augustin Fresnel, Thomas Young (1883–1829), A.H. Compton, Albert Einstein, Johann Jacob Balmer 2. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. The diameter of the nucleus is in the range of 1.75 fm (fm = femtometers, 1.75 x 10-15 m) for hydrogen, which is the approximate diameter of a single proton. The atomic radius of a hydrogen atom (including its electron cloud) is about 53 pm (pm = picometers, 5.3 x 10-11 m). Suppose the tennis ball at this station represents the nucleus of a hydrogen atom. How large would the atomic radius of a hydrogen atom be if its nucleus were the size of this tennis ball?