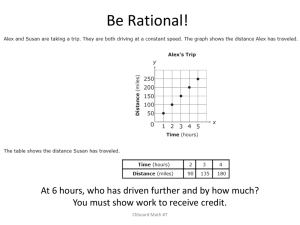
All the… small things Atoms and such
advertisement

All the… small things Atoms and such Brainstorm With your group: What do you know about atoms? What is a quark? Quarks are particles that make up parts of an atom (protons, neutrons, etc.) Up Quarks have a charge of + 2/3 Down Quarks have a charge of -1/3 neutron proton How big is a quark? A quark has a diameter of about 10-15 meters (one femtometer) This means to get the size of a quark, you need take 1/10 of a meter stick repeating 15 times! What about an electron? An electron is made of leptons. A lepton is a particle that does not experience the strong interaction (that is, the strong nuclear force). This is why electrons “orbit” the nucleus. A lepton is about 1/2000 the size of a proton. What is a real life representation of that relative size? So how big is a hydrogen atom? Hydrogen- diameter of 50×10−12 m (50 picometers) What is a physical representation that compares 50 picometers with a hydrogen nucleus that has the diameter of three quarks (3 x 10-15 m)? All of that extra “stuff ” in an atom is empty space!!! How big is the biggest atom? Cesium is the largest atom with a known size 260 picometers (260×10−12 m ) Compare that with the size of a hydrogen atom How does this ratio compare with hydrogen atom to quark ratio? Your assignment Develop visual model that: 1. Compares a hydrogen nucleus to a hydrogen atom 2. Compares that hydrogen atom with a grain of sand (one mm diameter). 3. Compares that grain of sand with a baseball (2.9 inch diameter). (the hydrogen nucleus should be represented with a visible object)