Excretion of Drug
advertisement

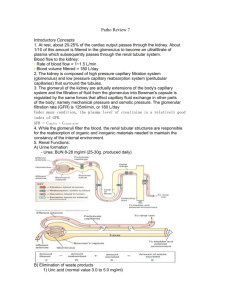
EXCRETION OF DRUG Dr. Muslim Suardi, MSi., Apt. Faculty of Pharmacy University of Andalas 2009 Excretion of Drug “Removal of the intact drug” Metabolites Drug molecules Renal Sweat Bile EXCRETION Nail Hair Saliva Other fluids Milk Excretion of Drug RENAL EXCRETION RENAL EXCRETION • • • • • • Nonvolatile drug Polar (easily removal) Water soluble Low MW, <300 Metabolites Slowly biotransformed by the liver Feces • Drug not absorbed by GIT • Bile excretion, no absorption in intestinal Biliary Excretion Drug Excretion Breast Milk Affect on baby Lung • Important in excretion of general anesthetic drug • Non polar • Gas • Drug/metabolites in small amount Tears • Example: Rifampicin • Important in drug information services to patients Saliva • Example: Excretion of Potassium Iodide Sweat • Smelly Kidney • Main excretory organ for removal of metabolite waste • Plays major role in maintaining the normal fluid & composition • Maintain salt & water balance: kidneys excretes electrolytes, water, waste product • Located in the peritoneal cavity • Nephrons: basic functional unit Nephrons • • • • Basic functional unit Responsible for the removal of metabolite Maintain of water & electrolyte balance Reabsorb of water by longer loops of Henle Kidney blood supply • Kidney is supplied by blood via the renal artery • Afferent arteriole carries blood toward a single nephron into glomerular portion of nephron (Bowman’s capsule) • Filtration of blood occurs in the glomeruli in Bowman’s capsule Kidney blood supply • From glomerulus, the blood flow out via the efferent arterioles • Then into a second capillary network that surrounds the tubules RENAL DRUG EXCRETION Include any combination of: • Glomerular filtration • Active tubular secretion • Tubular re-absorption Glomerular Filtration • About 180 L of fluid/day are filtered through the kidneys • Average urine volume is 1-1.5 L • Besides fluid regulation, the kidney also regulates the retention or excretion of various solutes & electrolytes Glomerular Filtration • Most small molecules are filtered through glomerulus from plasma • The filtrate contains some ions, glucose, & essential nutrients as well as waste products Glomerular Filtration • Waste products such as urea, phosphate, sulfate, & other substances • The essential nutrients & water are reabsorbed at various sites including the proximal tubule, loops of Henle, & distal tubules Glomerular Filtration • Both active reabsorption & secretion are involved • The urine volume is reduced • Urine generally contains a high concentration of metabolic wastes & elimination drug products Glomerular filtration • • • • Unidirectional process for most small MW Including: Non-ionized & ionized drugs Protein bound drugs (large), not filtered Pore diameter of glomeruli capillary: 70 nm • Hydrostatic pressure Renal Function “An indication of the state of the kidney & its role in physiology” Creatinine, urea, electrolytes, & inulin were used to determine renal function. These measures are adequate to determine whether a patient is suffering from kidney disease. GFR • To know renal patients • GFR is measured by using a substance that is eliminated by filtration only • The substance is neither reabsorbed nor secreted Glomerular Filtration Rate • Inulin & Creatinine • Clearance of inulin will be equal to the GFR (125-130mL/min) Calculation of GFR • By comparing urine creatinine levels with the blood test results. • It gives a more precise indication of the state of the kidneys. Inulin • • • • • Polyvalent carbohydrate No protein binding No secretion into tubule No reabsorption from tubule Excretion into urine merely by glomeruli filtration • Plasma inulin is excreted by filtration only & no reabsorption Glomerular filtration • The value for the GFR correlates fairly well with BSA • GF of drugs is directly related to the free drug concentration in plasma • As the free drug concentration in plasma increases, the GF for the drug will increase proportionally GFR • The GFR is expressed in mL/min. • For most patients, a GFR over 60 mL/min is adequate. • GFR measurements can aid a nephrologist in deciding when to initiate dialysis or renal transplantation. Corrected GFR • Very often, the GFR is expressed as ml/min/1.73 m2. • GFR needs to be corrected for the BSA • Most adults have a BSA that approaches 1.7 (1.6-1.9), extremely obese or slim patients should have their GFR corrected for their actual BSA. • BSA can be calculated on the basis of weight & height. Ampicillin • • • • Excretion mainly via glomeruli filtration Therapeutic ratio: very wide May be excreted via bile GFR decrease, non renal excretion increase • No individualization in dosage regiment Furosemide • GFR decrease, non renal excretion increase • No individualization in dosage regiment Kanamycin • Excretion primarily by renal clearance • Narrow therapeutics index If Cl < 35 mL/min: • 1. Dose should be reduced • 2. Usage should be considered NOMOGRAM • Relationship between GFR & Dose • Avoid over dose • Calculation the proper dosage regimen A quick dosage regimen adjustments for patient with characteristics requiring adjustments such as: Age BW Physiology state NOMOGRAM • Some nomograms make use of certain physiologic parameters, such as serum creatinine concentration, to help modify the dosage regimen according to renal function • For marketed drugs, the manufacturer often provides tabulated general guidelines for use in establishing a dosage regimen for patient, including L & M dose NOMOGRAM • The result displayed diagrammatically on special scaled axes to produce a simple dose recommendation based on patient information Active Renal Secretion • A carrier-mediated system that requires energy input, because the drug is transported against a concentration gradient • Carrier system is capacity limited & may be saturated • Drugs with similar structures may compete for the same carrier system Active Renal Secretion • 2 active renal secretion systems: weak acids & weak bases • Ex: probenecid will compete with penicillin for the same carrier system (weak acid) • Active tubular secretion rate is dependent on renal plasma flow • Compound commonly used to measure active tubular secretion: PAH, Diodrast Active Renal Secretion • Both of substances are filtered by the glomeruli & secreted by the tubular cells • Active secretion is extremely rapid • All the drug carried to the kidney is eliminated in a single pass. • The clearance of these compounds, reflects the effective renal plasma flow (ERPF) • 425-650 mL/min Active Renal Secretion • Drug that is excreted solely by glomerular filtration, t1/2el may changed markedly in accordance with the binding affinity of the drug for plasma protein • In contrast, protein binding has very little effect on t1/2el of a drug excreted mostly by active secretion Active Renal Secretion • Because drug protein binding is reversible, the bound drug & free drug are excreted by active secretion during the first pass through the kidney. • Ex: Some of the penicillin are extensively protein bound, but their elimination half lives are short due to rapid elimination by active secretion. Tubular Reabsorption • Occurs after the drug is filtered through the glomerulus • Can be active or passive • If a drug is completely reabsorbed (eg. glucose), then the value for the clearance of the drug is approximately zero • For drugs that are partially reabsorbed, clearance value will be < GFR of 125-130 mL/min Tubular Reabsorption • Reabsorption of drugs that are acids or weak bases is influenced by the pH of the fluid in the renal tubule (ie. urine pH) & pKa of the drug. • Both of these factors together determine the % of ionized & unionized drug • Generally, unionized species is more lipid soluble & has greater membrane permeability Tubular Reabsorption • The unionized drug is easily reabsorbed from the renal tubule back into the body • This process of drug reabsorption can significantly reduce the amount of drug excreted, depending on the pH of the urinary fluid & pKa of the drug • The pKa of the drug is constant, but the normal urinary may vary from 4.5 to 8.0 Tubular Reabsorption • Variation of urinary pH depending on: 1. Diet 2. Pathophysiology 3. Drug intake Higher urine pH caused by: • Vegetable diets Lower urinary pH caused by: • Diets rich in carbohydrates • Diets rich in protein Drug • Ascorbic acid & NH4Cl may decrease the urine pH • Antacid (Na2CO3) may increase the urinary pH • I.V fluids, such as solutions of HCO3- or NH4Cl, are used in acid-base therapy. • Excretion of these solution may drastically change urinary pH & alter drug reabsorption & drug excretion Tubular Reabsorption • % of ionized weak acid drug corresponding to a given pH can be obtained from the Henderson-Hasselbalch equation Tubular Reabsorption Henderson-Hesselbalch Equation For weak acids [ ionized drug ] pH = pKa + log ______________ [ unionized drug ] Tubular Reabsorption • The extent of ionization is more greatly affected by changes in urinary pH with a pKa of 5 than with a pKa of 3 • Weak acids with pKa values < 2 are highly ionized at all urinary pH values & are only slightly affected by pH variation Tubular Reabsorption Henderson-Hesselbalch Equation for weak base [ unionized drug ] pH = pKa + log ______________ [ ionized drug ] Tubular Re-absorption • The greatest effect of urinary pH on reabsorption occurs with weak base drugs with pKa values of 7.5 - 10.5 Tubular Re-absorption • From the Henderson-Hesselbalch relationship, a concentration ratio for the distribution of a weak acid or basic drug between urine & plasma may be derived Tubular Re-absorption Urine-plasma ratio for weak acids: (pH urine – pKa) 1+ 10 U/P = ------------------------1 + 10 (pH plasma – pKa) Tubular Reabsorption Urine-plasma ratio for weak bases: (pKa - pH urine) 1+ 10 U/P = ------------------------(pKa pH plasma) 1 + 10 Amphetamine • Weak base • Will be reabsorbed if the urine pH is made alkaline & more lipid-soluble non-ionized species are formed Amphetamine • In contrast, acidification of the urine will cause the amphetamine to become more ionized (form a salt) • The salt form is more water soluble & less likely to be reabsorbed & has tendency to be excreted into the urine more quickly Salicylic acid • Weak acid • Alkalination of the urine causes more rapid excretion of the drug Clinics • Weak base drug will be excreted or will not be absorbed in big amount in acidic urine • Weak acid drug will be excreted rapidly in alkaline urine • In clinics: important in barbiturate toxicity treatment. Barbiturate a weak acid with pKa of 7.2 • The ratio of ionized form may be change by urine pH modification DRUG CLEARANCE • A pharmacokinetic term for describing drug elimination from the body without identifying the mechanism of the process • “Vol of fluid clear of drug per time unit” DRUG CLEARANCE • The fixed vol of fluid (containing the drug) cleared of the drug per unit of time • The units of clearance are vol/time (mL/min, L/hr) • Ex: if Cl זof penicillin is 15 mL/min in a patient & penicillin has a Vd of 12 L, then from the definition, 15 mL of the 12 L would be cleared of drug per min DRUG CLEARANCE • Alternatively, Cl זmay be defined as the rate of drug elimination divided by the plasma drug concentration • The volume of plasma eliminated of drug per unit time • Practical way to calculate clearance based on plasma drug concentration data BILLIARY EXCRETION BILLIARY EXCRETION • An important system for the secretion of bile & excretion of drugs • The common bile duct empties into the duodenum • Bile primarily consists of water, bile salts, bile pigments, electrolytes, & to a lesser extent, cholesterol & fatty acids BILLIARY EXCRETION • Molecular weight & excretion • > 500 mainly excreted in the bile • 300-500, excreted both in urine & bile. For these drugs, a decrease in one excretory route results in a compensatory increase in excretion via other route • < 300, almost exclusively excreted via kidney BILLIARY EXCRETION • Drugs excreted into bile are metabolites, very often glucuronide conjugates • Formation of a glucuronide increases the MW by the nearly 200, as well as increasing the polarity • Excreted into bile: digitalis glycosides, bile salts, cholesterol, steroids, indomethacin BILLIARY EXCRETION • Compounds that enhance bile production stimulate billiary excretion of drugs normally eliminated by this route • Phenobarbital: may stimulate the billiary excretion of drug • In contrast, compounds that decrease bile flow or pathophysiologic conditions that cause cholestasis will decrease billiary drug excretion BILLIARY EXCRETION • The route of administration may also influence the amount of the drug excreted into bile • Drugs given orally may be excreted by the liver into the bile to a greater extent than if the drugs are given iv-ly Enterohepatic circulation Drug in faeces • Oral: due to billiary excretion or incomplete absorption? • Parenteral: Drug was excreted in the bile! Enterohepatic circulation “ The cycle in which the drug is absorbed, excreted into the bile, & reabsorbed in duodenum” • Some drugs excreted as a glucoronide conjugate will become hydrolized in the gut back to the parent drug by the action of a Beta-glucuronidase enzyme present in the intestinal bacteria. Parent drug becomes available for reabsorption