Acids & Bases 1
advertisement

Acid and Base Equilibria
Chapter 8
Acid and base definitions
based on experiments.
Acids
Tastes sour
Conducts electricity
Changes the colour of litmus paper from blue to
red
Turns neutral (green) Bromthymol blue to yellow
Reacts with active metals such as zinc and
magnesium, liberating hydrogen gas (H2).
Reacts with carbonates, releasing carbon dioxide
gas (CO2)
Bases
Tastes bitter
Conducts electricity
Changes the colour of litmus paper from red
to blue
Turns neutral (green) Bromthymol blue to
blue
Turns colourless phenolphthalein to pink
Reacts with an acid to destroy its properties
Feels slippery
Arrhenius Theory Review
Acids are solutes that produce hydrogen ions/protons
in aqueous solutions (increases the concentration of
H+) or hydronium ions (H3O+). Ex:
H+(aq) + Cl-(aq)
OR H2O(l) + HCl(g)
H3O+ (aq) + Cl-(aq)
Bases produce hydroxide ions when dissolved in
water.
HCl
NaOH(s) Na+(aq) + OH-(aq)
However, this model does not account for basic
properties of compounds that do not contain
hydroxide ions, such as ammonia –NH3(aq)
{NH3(g) + H2O(l)
NH4 +(aq) + OH-(aq)}
Bronsted-Lowry Theory
According to this theory, an acid is a proton donor, and a base is
a proton acceptor. A substance can only be classified as one or
the other for a particular reaction (as it can change from one
reaction to another). focus on proton transfer
Ex. of acid: HCl; when hydrogen chloride reacts with water, a
proton is transferred from HCl to H2O
H2O + HCl(g)
H3O+ (aq) + Cl-(aq)
base acid
conj. acid conj. base.
Ex. of base: when ammonia reacts with water, water now acts
as an acid because it donates a proton to ammonia, which is the
Bronsted-Lowry base
NH3(g) + H2O(l)
NH4 +(aq) + OH-(aq)
base
acid
conj. acid conj. base.
Amphoteric
As you can see in the above reactions, water can act
as either a base or as an acid. A substance that can
act as either a Bronsted-Lowry acid or B-L base given
the reaction is called amphoteric (amphiprotic); it can
donate or accept a proton.
{amphoteric: may act as an acid or base;
amphiprotic: may accept or donate protons; for
Bronsted-Lowry acids and bases, amphiprotic is
always amphoteric, but not in more general
definitions}; equilibrium conditrion; like water or
bicarbonate ion in baking soda
Amphoteric – Water
Another example of water as amphoteric:
HCO3-(aq) + H2O(l)
(l)
base
acid
HCO3-(aq) + H2O(l)
H3O+(aq)
acid
base
(autoionzation)
H2CO3(aq) + OH-
CO32-(aq) +
Neutralization
benefit of B-L rather than Arrhenius is that it defines
acids and bases in terms of chemical reactions, so
that neutralizations do not have to produce water
and salt, as according to Arrhenius
Arrhenius neutralization: acid-base neutralization
produces water and salt as in
NaOH(aq) + HCl(aq) H2O(l) + NaCl(aq)
NH4OH(aq) + HCl(aq) H20(l) + NH4Cl
Acid-base neutralization without hydronium ions,
hydroxide ions, or water:
NH3(g) + HCl(g) NH4Cl
(proton transferred from Cl atom to N atom)
Reversible Acid-Base Reactions
Prompt:
Which is the
acid? How do
we know that?
Where is the
proton
transfer?
(Equilibrium implied in Bronsted-Lowry reaction)
In each proton transfer reaction at equilibrium, both forward and reverse reactions
involve Bronsted-Lowry acids and bases. On each side of the reaction are acids and
bases, which are called conjugate acid-base pairs.
For ex.:
HC2H3O2(aq) + H2O(l) C2H3O2-(aq) + H3O+(aq)
acid
base
conj base
conj acid
In any acid-base equilibrium, there will always be two acids and two bases.
The base on the right (product) is formed by the removal of the proton from the acid
on the left.
The acid on the right (product) is formed by the addition of a proton to the base on
the left.
A conjugate acid-base pair is a pair of substances whose molecular formula differ by
a single H+ ion (proton). ( the acid has one more proton than the base)
The acid in the forward reaction is a proton donor and the base is the proton
acceptor.
In the reverse reaction, the conjugate acid is the proton donor and the conj. base is
the acceptor.
Competition for protons
(p.530)
View acid-base reactions as a competition for protons between
two bases.
Strong acids in reactions go to almost 100% ionization or
percent reaction. (the proton transfer is almost a complete
forward reaction – and almost no reverse proton transfer
occurs); therefore, an equilibrium is not established in this case
Weak acids have a lower % ionization, so their equilibrium
position favours the reactants rather than the products.
The stronger an acid, the weaker its conjugate base – the acid
has a weaker affinity for the proton, and easily loses/transfers
the proton to its conjugate base.
The weaker an acid, the stronger its conjugate base. The
stronger the base, the stronger the attraction for protons.
Strong and weak acids
Bronsted-Lowry explanation of strong and weak acids:
HA(aq) is used as general symbol for acid, and A-(aq) as its
conjugate base.
Ionization reaction:
HA(aq) + H2O(l)
A-(aq) + H30+(aq)
The strength of the acid HA is determined by the extent of the
proton transfer.
The ionization equation of an acid in water is often abbreviated:
HA(aq) + H2O(l) A-(aq) + H30+(aq)
to
HA(aq) A-(aq) + H+(aq)
continued
For ex, if we go back to our previous example,
HC2H3O2(aq) + H2O(l)
C2H302-(aq) +
H30+(aq)
acid
base
conj base conj acid
then it reduces to
HC2H3O2(aq) C2H302-(aq) + H+(aq)
Although this reduced equation shows the change
that takes place, it does not demonstrate the
important role that water plays in causing the acid to
ionize, or that the proton most likely exists as a
hydronium ion (H3O+).
Strong Acids (p.534 )
an acid that is assumed to ionize quantitatively (completely) in aqueous
solution (100 % ionization is > .99% - however, we assume it’s 100%
in calculations)
hydrochloric acid HCl(aq), hydrobromic acid HBr(aq), sulfuric acid
H2SO4(aq), nitric acid HNO(aq), phosphoric acid H3PO4(aq)
Monoprotic acid: an acid that possesses only one ionizable (acidic)
proton; ex. HCl
Diprotic acid: an acid that possesses two ionizable (acidic) protons; ex.
H2SO4
Triprotic acid: possesses three ionizable hydrogen atoms; ex. H3PO4
Note: Dissociation: Chemistry
a. The process by which the action of a solvent or a change in physical
condition, as in pressure or temperature, causes a molecule to split into
simpler groups of atoms, single atoms, or ions.
b. The separation of an electrolyte into ions of opposite charge.
Strong Bases (p.537)
an ionic substance that (according to Arrhenius) dissociates
completely in water to release hydroxide ions
dissolve in water (dissociate completely)
LiOH(s), NaOH(s), KOH(s), RbOH(s), CsOH(s)
Mg(OH)2(s), Ca(OH)2(s), Ba(OH)2(s), Sr(OH)2(s)
For every mole of metal hydroxide that is dissolved, one mole of
hydroxide ion is produced.
NaOH(s) Na+(aq) + OH-(aq)
Group 2 elements – dissolve in water and form 2 hydroxide ions
Ba(OH)2(s) Ba2+(aq) + 2OH-(aq)
Hydroxide ions react with hydrogen ions – shifts equilibrium to
right, causing undissolved salts to dissolve and produce higher
hydroxide ion concentrations
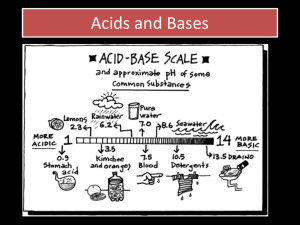
Hydrogen Ion Concentration and pH
(p. 540)
There is a wide range of concentration of hydrogen
ions and hydroxide ions in acidic and basic solutions.
The pH scale was developed to measure hydrogen
ion concentration.
pH = –log[H+(aq)]
Example:
Calculate the pH of a solution with a hydrogen ion
concentration of 5.3 x 10-9.
pH = –log [H+(aq)]
pH = –log (5.3 x 10-9) (two sig digits)
pH = 8.28
The solution has a pH of 8.28.
(p. 541)
pH of pure (neutral) water and any
neutral solution at SATP is 7.00 as the
hydrogen and hydroxide ions are equal.