Test Review
advertisement

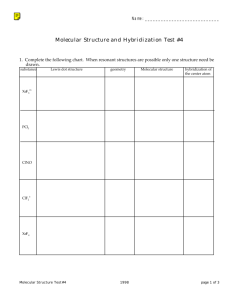
Chapter 7, 8, and part of 9 Study Guide 1. Answer with the appropriate label and name or formula Ionic or Name Molecular Chemical Formula (covalent) Lithium chloride Magnesium nitride nitrogen trifluoride Lead (II) sulfide dihydrogen monosulfide Aluminum sulfide Beryllium iodide dihydrogen monoxide carbon tetraiodide Chemical Formula Ionic or Molecular (covalent) Name Mn2S3 Ag2O C2Br4 KBr MgS CoN C3F8 CaF2 CuF 2. Identify the following properties as ionic or molecular (covalent) C) Covalent Compound I) Ionic Compound ___1. the strongest bond ___7. High melting points ___2. conducts electricity when dissolved in water or melted ___ 8. 2 or more nonmetals ___3. Carbohydrates, nucleic acids, and proteins ___9. Metal and nonmetal ___4. Alternating positive and negative particles ___10. Crystal lattice structure at room temperature ___5. Sharing of one e- pair between two atoms ___11. Transfer of electrons from one atom to another ___6. Low melting points ___12. Liquids and gases at room temperature 3. For the following molecules draw the Lewis Dot Symbols and circle the octet that every element has. (Hydrogen of course only has two) Example: A) C2H6 4. B) N2 C) H2O D) NH3 E) H2CO F) SiF4 Complete the Lewis Dot Structure of the following Molecular (covalent) compounds. Label electronegativitys with δand δ+. Label the molecular (covalent) compounds as Polar or Nonpolar. CO2 PCl3 NH3 O2 H2O H2CO