Syllabus for Pre-AP Chemistry
advertisement

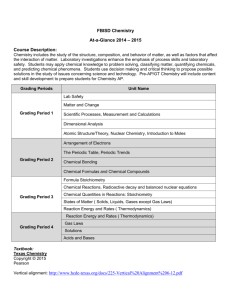
Syllabus AP Chemistry 2015-2016 OVERVIEW: AP Chemistry is designed to provide the student with the equivalent of an introductory first year course sequence in college chemistry. The course is designed for college-bound students who either would like to earn college credit (by AP examination) or would like to prepare for college chemistry while in high school. This is accomplished through an intensive, in-depth approach. As many colleges recommend, the minimum time required for outside class work is 1.5 hours per class hour, i.e. a minimum of 5 hours of homework per week. If you choose to take the College Board's Advance Placement test in Chemistry. The score on this test will be evaluated by the College Board and the scores reported to the colleges of the student's choice. Scores of one and two on the test will generally not qualify the student to receive credit. Scores of three, four, or five (the maximum score possible) will generally allow the student to place out of freshman courses. The amount of credit granted varies by grade and by university. The grade earned in the class is independent of the Advance Placement test. The laboratory portion of this class is to be the equivalent of a college laboratory experience. Because some colleges require proof of the laboratory portion of the course before granting credit, all students will keep a laboratory notebook. Some of the laboratory investigations may require the students to spend time in tutorials outside of school. Because of the nature of this course and the amount of new material that will be covered, a strong preparatory background in Chemistry-I is a requirement. The topics that you should be familiar with already include: atomic structure electron configurations bonding VSEPR theory acid-base chemistry nuclear chemistry chemical names and formulas chemical reactions (balancing and completing) periodicity behavior of gases mole concept mole calculations stoichiometry making and interpretation of graphs making observations from laboratory situations The nature of Chemistry requires the student to know certain basic facts that MUST be committed to memory. Mathematics is an integral part of this class. Problem solving strategies will be stressed throughout the year and this course also requires the student be able to solve problems WITH and WITHOUT a calculator. BIG IDEAS: 1. Structure of matter 2. Properties of matter – characteristics, states, and forces of attraction 3. Chemical Reactions 4. Rates of chemical reactions 5. Thermodynamics 6. Equilibrium MATERIALS NEEDED: The following are the materials needed for this class. 1. Loose-leaf notebook paper 2. Graph paper 3. 3 ring binder (1.5-2 inch, NOT 3 inch or bigger) 4. 1 pack of dividers 5. Scientific Calculator (a graphing calculator is fine!) – you may use mine if you need to. 6. Black or blue pens and #2 pencils with erasers. 7. 1 box of kleenex TEXTBOOK: Chemistry: A Molecular Approach, Nivaldo J Tro, 3rd Edition (Pearson Publishing), 2014 GRADING: A typical six weeks grading period would have: tests , major projects 50%, laboratory investigations 20% quizzes 20% daily grades 10% Within a grading period there will be generally no more than three major tests, and several short quizzes. One of the tests will be in AP format and will be graded according to AP standards. The grading procedures for this format will be explained before the students take the exam. Grading policies are more stringent for an AP course than a regular class. It is imperative that you put in the effort initially and avoid having to take measures to improve a grade after the fact. Major projects will have ten points deducted per day late. Daily assignment will be thirty points off the first day it is late, and you have 1 week to turn it in. Laboratory investigations and write ups will be lengthy and cannot be made up for a better grade. Low test grades will be discussed on a case by case basis. Tutorials will be required to address any make up work to improve your average. YEAR AT A GLANCE: 1st six weeks 2nd six weeks 3rd six weeks 4th six weeks 5th six weeks 6th six weeks Review of Chemistry-I, Laboratory Investigations, Solution Chemistry. REDOX Reactions, Nuclear Chemistry, and Thermochemistry. Thermodynamics, Kinetics, and Gas Laws. Equilibrium, Acid-Base Chemistry Chemical Equations, Electrochemistry Electron Configuration and Periodicity TUTORIALS: I am available mornings before school, 7:30 until the bell rings, and afternoons until 4:00, except on game days. Please communicate ahead of time if you are planning to attend tutorials in case I need to prepare materials for you or I have another conflict. If I am not in my room at the moment you arrive, I may have stepped out to the office or copy machine, so please wait or make use of your time with another teacher until I can return.