1
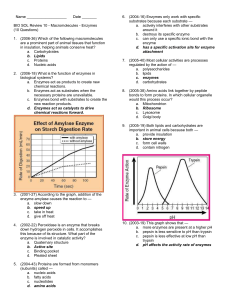
THE BUILDING BLOCKS OF
LIFE
Mrs. Geist, Biology
Swansboro High School, 2010-2011
Warm-Up
2
What does the term “organic” mean to you?
Can you name foods rich in:
carbohydrates/sugars
lipids/fats,
proteins?
and
Organic vs. Inorganic
3
Organic compounds: typically contain carbon (C)
and are associated with life
Inorganic compounds: usually do not contain
carbon and are associated with non-living things
Carbon is the building block of all living things!!
Carbon
4
Has 4 electrons in its outermost shell
4
electrons to share
Forms covalent bonds (shares electrons)
Single, double, or triple bonds
Atoms of nitrogen (N), oxygen (O), and phosphorus
(P) attach to carbon chains.
Macromolecules—giant molecules
5
4 major classes:
Carbohydrates
Lipids
Proteins
Nucleic
acids
Polymers- complex molecules
Monomers- small molecules, single units
Carbohydrates
6
Made of carbon (C),
hydrogen (H), and
oxygen (O)
Ex: sugars and
starches
2 H: 1 O
Major source of
energy
Carbohydrates: Simple Sugars
7
Simple sugars
(C6H12O6) smallest
carbs.
A.k.a.
monosaccharides
Ex: glucose,
fructose, galactose
Cell’s main source
of energy
Carbohydrates: Disaccharides
8
Disaccharide
(C12H22O11)- two
monosaccharides
together
Ex:
sucrose,
maltose, lactose
Carbohydrates (cont’d.)
9
Polysaccharides- many monosaccharides
4 major classes of polysaccharides:
1.
2.
3.
Starch- plant storage of glucose
Glycogen- animal storage of glucose
Cellulose- plants use for structural support
4.
Most abundant organic molecule on Earth
Indigestible bulk (or “fiber”)
Chitin- animals use for support, insect skeletons
2nd most abundant organic molecule on Earth
Warm-Up 8/31
10
In your own words, what is an organic
macromolecule?
What do the terms saturated and unsaturated fat
mean to you?
Where might we find protein in the body?
Lipids
11
Made up of carbon (C), hydrogen (H), and oxygen
(O)
Functions:
Store
energy
Provide insulation
Important parts of cell membranes (phospholipids)
Waterproof coverings (waxes, ex: bird feathers)
Not
soluble in water
Ex: fats, oils, waxes, steroids, phospholipids
Lipids (cont’d.)
12
Structure:
3
fatty acids
Long
chains of C with H
attached
1
glycerol
Alcohol
with a hydroxyl
(-OH) group on each of
its 3 C atoms
Dehydration synthesis:
attaches these parts
Removal
of water (H2O)
Lipids: Saturated vs. Unsaturated
13
Saturated
Unsaturated
Solid at room
temperature
Liquid at room
temperature
Contains lots of H
Contains less H
Single bonds between
C atoms
1+ double bonds
between C atoms
Proteins
14
Made up of nitrogen (N), carbon (C), hydrogen (H),
and oxygen (O)
Monomer: amino acids
Structure:
Amino
group (-NH2) on 1 end
Carboxyl group (-COOH) on the other end
Side chain (or R group) that differs for each of the 20
amino acids
Proteins: Amino Acids
15
Structure of an amino acid (monomer)
Proteins: 4 levels of organization
16
1.
2.
Primary: sequence of amino acids
Secondary: the amino acids within a chain can be
twisted or folded
3.
4.
Ex: alpha helices, beta pleated sheets
Tertiary: the chain itself is folded
Quaternary: If the protein has >1 chain, each
chain has a specific arrangement in space
Protein Functions
17
Some proteins control the rate of reactions and
regulate cell processes (called enzymes).
Act
as catalysts- speed up chemical reactions
some form bones, muscles, skin, and ligaments.
Others transport substances into/out of cells
Help to fight disease.
Enyzmes as catalysts
Enzymes are proteins that
act as biological catalysts.
Catalyst- speeds up the
rate of a chemical
reaction.
Speed up reactions that
are too slow or have
activation energies that
are too high to make them
practical for living tissue.
Enzymes act by lowering the
activation energy.
19
Functions of Enzymes
Regulating chemical
pathways.
Making material that
cells need.
Releasing energy.
Transferring
information.
Enyzmes as catalysts
For a chemical reaction
to take place, the
reactants must collide
with enough energy to
break existing bonds
and form new bonds
If reactants do not have
enough energy, no
reaction will take place.
Enzymes
Enzymes are very specific,
generally catalyzing only
one chemical reaction.
For this reason, part of an
enzyme’s name is usually
derived from the reaction
it catalyzes. Add –ase
ending.
I.e. lipase
I.e. proteinase
The Enzyme-Substrate Complex
Enzymes provide a site where reactants can be brought together to
react, reducing the energy needed for reaction.
The reactants are known as substrates.
The enzyme and substrates remain bound together until the reaction is
done and the substrates are converted to products.
The products of the reaction are released and the enzyme is free to
start the process again.
The cell theory states that new cells are produced from
nonliving material.
existing cells.
cytoplasm.
animals.
Copyright Pearson Prentice Hall
The person who first used the term cell was
a. Matthias Schleiden.
b. Lynn Margulis.
c. Anton van Leeuwenhoek.
d. Robert Hooke.
Copyright Pearson Prentice Hall
An Enzyme-Catalyzed Reaction
Substrates: glucose and ATP
Enzyme: hexokinase
Substrates bind to enzyme,
forming an enzymesubstrate complex.
The fit is so precise that the
active site and substrates
are often compared to a
lock and key.
Regulation of Enzyme Activity
Enzymes work best at certain pH
and temperature ranges and
can be affected by such
changes.
Many enzymes are affected by
changes in temperature.
Cells can regulate the activities
of enzymes.
Most cells contain proteins that
help to turn key enzymes “on”
and “off” at critical stages in the
life of the cell.
Nucleic Acids
Function: store and
transmit hereditary, or
genetic, information.
2 kinds of nucleic acids:
1. ribonucleic acid (RNA)
2. deoxyribonucleic acid
(DNA).
Nucleic Acids
29
Monomer = nucleotides, which are composed of 3
parts:
5-carbon
sugar- deoxyribose (DNA) or ribose (RNA)
Nitrogen base- adenine (A), thymine (T), cytosine (C),
guanine (G), uracil (U)
Phosphate group- contains phosphorus (P) and oxygen
(O)
2 Types:
Deoxyribonucleic
Acid (DNA)
Ribonucleic Acid (RNA):
Deoxyribonucleic Acid (DNA)
30
Double helix (spiral)
Stores genetic information
Ribonucleic Acid (RNA)
31
single
helix
Plays a role in
manufacture of
proteins
Enzyme (speed up
reactions)
Nucleotides
32
DNA and RNA
Adenine
(A)
Guanine (G)
Cytosine (C)
DNA only
Thymine
(T)
RNA only
Uracil
(U)
3 parts of a nucleotide
33
5-carbon sugar (deoxyribose in DNA or ribose in
RNA)
Nucleotide base
Phosphate group
The DNA and RNA Strand
5'
Base
Nucleotide + Nucleotide(n)
5'
1'
3'
= DNA (or RNA) strand
Base
Base
3'
2005 VisiScience Corporation. All Rights Reserved.
Does temperature affect an enzyme
reaction? (pp. 164-165)
Problem: Does the enzyme peroxidase work in
cold temperatures?
Does
peroxidase work better at higher
temperatures?
After being frozen or boiled?
Hypothesis: “If…, then …” statement.
Materials: clock, beakers, kitchen knife, tongs,
potato, ice, hot plates, thermometers, 3%
hydrogen peroxide (H2O2), hot gloves
35
Peroxidase
2H2O2 2H2O + O2
Hydrogen Peroxide water + oxygen gas
Hydrogen peroxide is damaging to cells
Peroxidase speeds up the breakdown of
hydrogen peroxide
36
Planning Experiment
Hypothesis
Boiling, ice bath,
warm water bath,
room temperature
Steps to be taken
Add 1 drop H2O2 to
the potato slice and
observe what
happens
What data will you
collect? How will you
record them?
What factors should
be controlled?
How will you achieve
those temperatures?
Carry out the expt.
37