Chemistry I LRP - Greenwood County School District 52
advertisement

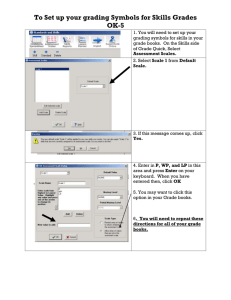
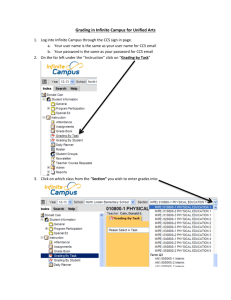
GREENWOOD COUNTY SCHOOL DISTRICT 52 LONG-RANGE PLAN NAME: Tripp Henderson DATE: 19AUG15 ASSIGNED SCHOOL: Ninety Six High School II. List long-range LEARNING AND DEVELOPMENTAL GOALS for the students that you teach. Goals may be categorized as “General” (applicable to all students), and “Subject Area” (goals specific to a particular subject, group, etc). By the end of the semester, my Chemistry I students will: 1. Formulate a testable hypothesis based on literary research and previous knowledge. 2. Identify and select experimental variables and controlled conditions. 3. Design a scientific investigation based on chapter concepts. 4. Identify technologies that could enhance the collection of data. 5. Select appropriate safety equipment to conduct an investigation. 6. Describe the appropriate response to emergency situations in the laboratory. 7. Draw conclusions based on quantitative and qualitative data. 8. Perform dimensional analysis calculations. 9. Formulate and revise scientific explanations and models using logic and evidence. 10. Recognize and analyze alternative explanations and models. 11. Trace the historical development of the model of the atom. 12. Cite the physical and chemical evidences for the existence and structure of the atom. 13. Compare and contrast the component particles of the atom. 14. Trace the development of nuclear models. 15. Identify the charge, component particles, and relative mass of the nucleus. 16. Explain what an isotope is, including the stability of it. 17. Compare and contrast fission and fusion reactions. 18. Explain nuclear decay. 19. Predict the charge of an element based on its outer electron arrangement. 20. Trace the development of the periodic table. 21. Interpret the information that is present on the periodic table. 22. Determine atomic number, mass number, number of protons, number of neutrons, and the number of electrons. 23. Compare and contrast elements and compounds. 24. Write and name chemical compounds. 25. Distinguish between ionic and covalent bonds. 26. Analyze the chemical and physical properties of substances. 27. Compare and contrast solids, liquids, and gases based on particle arrangement and the energy that binds them together. 28. Name and write organic compounds. 29. Balance chemical equations. 30. Differentiate between acids and bases. The additional goals for my class are: 1. To improve presentation skills through the use of and creation of multimedia projects. 2. To use a learning style that is not their preference. 3. To write about scientific concepts effectively and with clarity. 4. To read and critique scientific articles (about concepts addressed in the standards) and make connections to what is happening in the world today concerning these topics. 5. To use the internet to find information and use that information to enhance learning. III. In the space below, and using additional sheets as necessary, provide your instructional unit plans for the year. Plans should include all units you intend to teach, the sequence in which they are to be taught, and an approximate timeline for teaching them (by month, grading period, or etc.). You may use any format you desire for developing the instructional unit plans. First Nine Weeks Week 1 Chapter 3 Atoms: The Building Blocks of Matter Week 2 Chapter 4 Chapter 5 Arrangement of Electrons in Atoms The Periodic Law Week 3 Chapter 6 Chemical Bonding Week 4 Chapter 7 Chemical Formulas and Chemical Compounds Week 5 Chapter 20 Carbon and Hydrocarbons (simple ones) Week 6 Chapter 8 Chemical Equations and Reactions Week 7 Chapter 19 Oxidation and Reduction Reactions Week 8 Chapter 9 Stoichiometry Week 9 Chapter 9 Stoichiometry Second Nine Weeks Week 10 Chapter 13 Solutions Week 11 Chapter 15 Acids and Bases Week 12 Chapter 16 Acid-Base Titration Week 13 Chapter 21 Other Organic Compounds Week 14 Chapter 10 Physical Characteristics of Gases Week 15 Chapter 11 Molecular Composition of Gases Week 16 Chapter 12 Liquids and Solids Week 17 Chapter 17 Reaction Energy and Reaction Kinetics Week 18 Review and Exam IV. List the Key materials and resources needed to attain the goals and teach the units you have listed in your long-range plan. Provide information on ordering these key materials and/or lining up these resources. Please DO NOT make this a “laundry list” of routine classroom supplies. The key materials and resources used in the classroom are: The South Carolina Science Curriculum Standards, the textbook, Modern Chemistry (Holt, Rinehart and Winston - publisher), workbooks, internet, and laboratory materials and equipment. Additional materials needed and requested to adequately enhance instruction and student learning: 1. 2. 3. 4. 5. 6. 7. 8. 9. Chemicals Index Cards Colored pencils Models White Board LCD Projector Promethean Board Construction Paper Laptop Computer I will obtain internet permission forms from the Media Specialist. For those items that are related specifically to the subject area, I will put in all requests to the Science Department Chair and speak to the principal about funding all other items that I feel will enhance the learning of the students. The LCD Projector and Promethean Board are stationary items in the room that are used to enhance instruction and student learning. V. Provide your plan for assessing, evaluating, and recording students’ progress and achievement. Be sure to include assessment strategies, and evaluative criteria and procedures for maintaining records. Be specific as to how grades are computed. All students’ grades will consist of chapter tests, quizzes, lab reports, lab participation, projects (which will include preparing a Power Point presentation), homework, classwork, and outside readings as assigned. Grading Policy 1. Chapter Tests – each test will count three times. 2. Quizzes – each quiz will count two times. 3. Lab Reports – each lab report will count two times. 4. Homework – all homework assignments will count one time. 5. Classwork – all classwork assignments will count one time. 6. Outside Readings – all outside readings will count one time. 7. Lab Participation – will count one time. A point system will be used to determine each student’s average at the end of each nine weeks. Each assignment will be assigned a point total. All tests will be assigned a point value of 100 points. However, there may be times when the point total for a test will exceed 100 points. The point total for quizzes will vary but will not exceed 50 points. Projects will be assigned a point value of 100 points. Lab reports will be assigned a point value between 20-40 points. Lab participation will be assigned a point value of 25 points. Both homework and classwork will be assigned a point value between 10 – 35 points. All outside readings will be assigned a value of 25 points. To compute the grade at the end of each nine weeks, each assignment will be multiplied by the appropriate number (times that each counts) and added with all other assignments to obtain the total number of points earned. The total points earned will be divided by the total points given and multiplied by 100 to convert the grade to the standard grading scale. The following grading scale will be used to determine the letter grade that will be assigned: 93 – 100 = A 85 – 92 = B 77 – 84 = C 70 – 76 = D 0 – 69 = F All grades are kept on the computer in the classroom using the Power School computer software. This computer program keeps a running total and average of all grades for each student. It also allows for individual grade reports to be printed for students and parents. In addition, the grades are also saved to a computer disc as a safeguard and a backup, as well as hard copies printed out at the end of each week. VI. List your rules and procedures for managing students. If you provide handouts to your students, those may be attached. If rules are attached, provide only comments for clarification in the space below. All rules and consequences stated in the Ninety Six High School Student Handbook will be honored and enforced in class. In addition to the rules outlined in the handbook, the following will also apply: 1. Students will come to class prepared. This means that students will come to class with all materials – book, notebook, pencil, pen, etc. If a student comes to class unprepared five points will be deducted from his/her participation grade. 2. Students will remain in their seats at all times unless directed otherwise by the teacher. Students will not be allowed to wander around the classroom unless they are participating in an activity that involves movement. 3. Students will remain in their seats until the dismissal bell signals that class has ended. 4. Students will remain quiet and seated at all times while the announcements are being made. 5. Students will not enter the lab area unless directed to do so by the teacher. Any student who is in the lab area without permission will have a ten minute after school detention with the teacher with an increase of ten minutes for each subsequent occurrence. If the problem becomes habitual, a parent conference will be requested with an administrator present. 6. Students will follow all safety rules and precautions while working in the laboratory area with chemicals and equipment. Horseplay in the lab will not be permitted. Students who do not follow the safety rules and precautions will be given a grade of zero and assigned an alternate assignment during the next lab. Students will be given a warning for all first offenses on the above stated rules except the rules that apply to entering the lab area and conduct in the lab. Students will be assigned a ten minute after school detention for the second offense and ten minutes will be added for each subsequent occurrence. A telephone call home will accompany each occurrence. After-school detention will be assigned up to a maximum of one hour. At this point, a conference will be requested with the parents and an administrator. VII. How do you communicate with parents? Please list/explain any methodologies you utilize to keep parents informed of their children’s progress. Communication is the key to building successful relationships with students and their parents. I feel that it is one of the keys that enables a child to be academically successful and that it makes a difference if the child should have difficulties in a class. It is also an effective way to let parents know about their child’s behavior. In addition, it gives me the opportunity to make parents aware of my expectations both academically and behaviorally. In addition, it helps me keep parents abreast of any successes, improvements, problems, or special needs that may arise. The ways that I inform parents of their child’s progress are as follows: 1. 2. 3. 4. 5. 6. 7. Open House Interim Grades/Interim Report Report Card Email Telephone calls Parent Conferences (as needed) Progress Reports (upon request) VIII. Briefly explain how you modify your LRP as changes occur which precipitate alterations of the plan. If changes to this LRP are necessary, I will make the changes and the corrections directly to the computer generated document, and distribute it to the principal and other appropriate administrators.