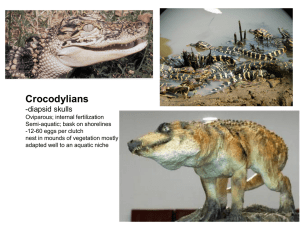
finaldissertation
advertisement

1.0 Introduction There are 23 recognised species of extant crocodilians (crocodiles, alligators, caiman and gharials), these can be split into three families: Alligatoridae (8 species; alligators and caimans), Crocodylidae (14 species; crocodiles and tomistoma) and Gavialidae (1 species; gharial) (IUCN CSG, 2010). Of the 8 species of Alligatoridae, there are four different genera: Alligator, Caiman, Melanosuchus, and Paleosuchus. In this study, the two genera researched were Caiman and Melanosuchus. Within the study site of the North Eastern Peruvian Amazon, there are three species of caiman, Caiman crocodilus, Melanosuchus niger and Paleosuchus trigonatus. However, no Paleosuchus trigonatus were seen throughout the research period and are consequently not considered in this study. Crocodilians’ existence is continuingly threatened due to the demand for their hides in the production of luxury items such as bags, wallets and belts. Crocodilians are also exploited for their meat. Trade in crocodilians is primarily an international matter as the items tend to be manufactured outside of the species’ range states; therefore the greatest challenge in their protection has been controlling the export of hides across international borders. The introduction of Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) in 1975 has proved very successful in controlling all trade in flora and fauna, in particular crocodilians. Habitat fragmentation and degradation puts further pressure on the three mentioned species in North Eastern Peru, along with the pollution of their habitat through mining for gold, which to some extent takes place in their ranges. All these threats have caused many species’ population numbers to decline, resulting in their addition to the International Union for Conservation of Nature (IUCN). 1.1 Protection of crocodilians Before the introduction of CITES, crocodilians had previously been neglected, their protection only came about when it was realised that the wide scale exploitation of crocodiles was resulting in the decline of the crocodilian population. The previous view held by many who lived in close proximity to crocodilians, that they were vermin had led to high levels of their slaughter being completely unrestricted by local authorities (Klemm; Navid, 1984). Hunting Caiman, for example, was legal in Brazil until 1967 when law no.5 197/67 banned all future hunting of wildlife; subsequently, the only caiman hides traded were ones from old stockpiles that had legal permission to be cleared. 1.2 Exploitation through trade Crocodilians have been traded since the early 1920s; uncontrolled commercial exploitation resulted in Melanosuchus Niger suffering a drastic population decline. Crocodilian skins first became used in fashion 1 during the 1920’s, when they were primarily associated with luxury items. However, as early as the 1930’s, the hides were already being increasingly used for mass produced items. Between 1945 and 1960 trade in crocodilian hides peaked (Thorbjarnarson, 1999i). During this period over three million skins were traded per year (Luxmoore, 1992). The hunting for crocodile skins was driven by its use in fashion, M.niger, one of the species researched in this study, was targeted for, what was considered, its superior skin. Towards the 1960’s, as the populations of M.niger were reaching a disturbing low level, hunting preferences turned to C.crocodilus, and by the late 1970’s, M.niger had suffered such a drastic population decline and had become rare. Crocodilian hides are split into two categories based on the quality of the leather: ‘classic’ and ‘nonclassic’. Those hides categorized as classic are of high value and low volume, whereas non-classic hides are of low value, and available in high volumes. These distinctions were made when trade in hides was at its peak, but the division is still apparent today (Luxmoore, 1992). Comparatively, the hide of C.crocodilus is inferior to that of M.niger due to the high level of ossification of the ventral skin; however, this can be rectified using a more complex tanning process (Magnusson 1982). Generally though, due to the high costs of this tanning process, unless availability of hides is scarce, the only part used commercially is the lateral flank region (Ross IUCN/SSC 2008). Between 1962 and 1969, at least 53,433 M.niger skins were exported from Peru; it was believed these all originated from individuals in the Amazon region. 101,641 hides from the C.crocodilus were exported from the same area within the five year period, from 1962 to 1967 (Smith, 1981). These figures show how the preference for C.crocodilus was on the increase, whilst that for M.niger was decreasing. From the beginning of trade in hides, the prices have gone through a ‘boom and bust cycle’; which demonstrates the vulnerability of any conservation program that relies on the sale of luxury items for project funding. The noteworthy change in prices from 1991-1992 has been associated with the poor world economy at this time, the increase of customer resistance to wildlife products, and the over supply of skins worldwide. During this time period, Brazilian caiman ranches were closed and harvest levels in Venezuela decreased (Thorbjarnarson, 1999ii). Since 1973, the export of caiman hides under 1.5 metres has been banned in Colombia. However, this control did not have the effect expected: fully 84% of the 556 422 hides exported were under the minimum legal length (Smith 1981). Although caiman and the trade of their skins is prohibited throughout Brazil, due to the large extent of the Amazon Basin in Brazil, the hunters access to the various local communities, and widely distributed transport systems, the policing of these laws and regulations is practically impossible (Magnusson, 1982). 2 For example, Leticia, a city on the Colombian Amazon, has become a major channel in exporting caiman hides. The hides are from species caught and killed in Peru and Brazil, which are then easily smuggled across the border. Reports have shown an estimated 400,000 caiman hides being sold from Leticia in this way (Smith, 1981). 1.3 Regulation of trade Species in tropical forests tend not to be managed as well as those in temperate areas; this is primarily due to the belief that hunting, as part of customary practices, is a traditional aspect of rural economies and culture. Also, due to these methods of hunting being sustainable in the past, it is still believed by many that they continue to have this effect (Bodmer, Robinson, 1999). Until 1975, all regulation of trade in crocodilians was executed at a local or national level; due to this the majority of all exported skins were taken from wild individuals. In 1975, the introduction of CITES ensured there was a stringent system in place to control their use and sustainability. CITES has been proved to have a considerable positive impact on international crocodilian trade (Luxmoore, et al.1985). CITES listings gradually reduced the supply of wild crocodilians involved in trade, and in turn, adapted its controls to respond to developments in other forms of crocodilian production. For example, Appendix II also includes ‘look-a-like’ species to ensure that species that are not protected are not hunted as a cause of their specimen’s apparent similarity to those species that are protected for established conservation reasons (MacGregor, 2006). In addition, by 1992, there were at least five levels of control, monitored by a variety of mechanisms, accorded to crocodilians under CITES (Luxmoore, 1992). i) Appendix I. ii) Appendix I, ‘bred in captivity for commercial purposes’. iii) Appendix II, transferred from Appendix I for ranching. iv) Appendix II, on the basis of an interim transfer from Appendix I and subject to quota. v) Appendix II. Ranching and quotas continue to be used as precautionary measures in management programs for crocodilians (Hutton et al, 2001). At least 30 countries are permitted under CITES to export crocodilians, from wild, ranched or captive-bred sources. Considering all this, CITES has been central to the gradual replacement of unregulated crocodilian exploitation with other forms of crocodilian production, notably ranching and captive-breeding. Other reasons for the shift from wild caught crocodilians to those captivebred include: scarcity of wild individuals, cultural preferences, conservation motives, and business 3 interests. Changes in the supply of crocodilian skins pre-CITES and currently have been well documented, a number of these are listed below. Factor Pre-CITES Current Source of supply Wild—virtually 100% Wild 6%, ranched 22%, captivebred 72% No. of skins in international 1-2 million (est’d.) 1 million trade Producers Hunters—independent dispersed Producers of wild skins and Mainly medium-to-large business interests Hunters—independent and Mix of independent hunters and dispersed collectives Prices per unit (for producer) Higher than now Lower than before Prices per unit (for retailer) Commensurate Commensurate Average quality Lower than now Higher than before Leather supply Higher than now Lower than before Average size of skin Larger than now Smaller than before Supply risk Less certainty of supply Far greater certainty of supply Market segmentation Species and caiman versus Quality, fashion and, to a lesser classics extent, caiman versus classics (Table 1: The differences in supply of hides pre-CITES and currently. MacGregor, 2002) During the first decade of CITES, problems with the accumulation of hides that had been collected prior to its implementation had to be overcome. Prior to 1984, CITES export statistics, not including illegal or undeclared trade, showed that less than 20% of the caiman hides in trade had even the potential to be legal (Thorbjarnarson, 1999). The introduction of CITES also meant that all crocodilian species were automatically allocated to Appendix I or II, excluding the specimens eligible for the personal-effect exemption, any export or re-export of items entirely or partially made of readily recognizable skin pieces of crocodilians requires an import permit and/or an export permit or a re-export certificate to be previously granted and presented, in accordance to Article III (2) and (4) and Article IV (2) and (5) of the Convention (CITES, 2007). Some nations argued the point that their native crocodilians had never been at an unstable or endangered level and, therefore, should never have been classified as Appendix I. This was 4 the case with some states in Africa and the Nile crocodile, Crocodilus niloticus. Those developing countries that the legislation affected found it difficult to raise the funds to ensure that the species’ population and recovery were well documented. The result of these disputes was that CITES created resolutions that loosened the requirements for legal trade: either the promotion of ranches; or the establishment of temporary CITES approved annual quotas (Thorbjarnarson, 1999). CITES main aim was to ensure that all exports were from a sustainable source. The wording of this provided a way around these regulations for many parties. The creation of ranches and the use of captive breeding enabled them to state that the crocodilians were from a sustainable source and therefore it was legal for them to be exported and traded. Currently, the control of the trade in crocodilian skins is considered to be very effective and has made it possible to eliminate the greatest part of a formerly flourishing illegal trade. In fact, it is widely recognized as CITES best success story (CITES, 2007). 1.4 Trade in meat Soon after the 1967 Brazilian law no.5 197/67 came into effect, the hunting of caiman specifically for their meat began, which was made easier by the increasing number of roads being constructed into more dense forest areas. These had primarily been built by national authorities, loggers and miners, and provided better access for rural communities, traders and hunters. When caiman are caught for meat, it is unlikely that the hunters are catching the meat for their own consumption. Often, the meat will be salted and preserved to cross the border where it will be sold on in Brazil and Colombia. When the meat is sold in Brazil, it is likely to be sold as what it is: caiman meat. However, in Peru and Colombia the meat will be sold on as paiche, one of the largest freshwater fish in the world, found only in South America (Da Silveira, R. Thorbjarnarson, J, 1999). The demand for paiche meat has proved to be somewhat detrimental to the recovery of M.niger: during the three year period, from 2001 to 2003, caiman meat was used as a copy of the valuable fish in Peru. This resulted in intensive hunting of the black caiman along the Yavari River in the Lago Preto Conservation Concession (Bodmer et al.2008). When hunting takes place for meat, the hunters tend not to be as audacious with their hunting destinations as they would be if they were hunting caiman for their skins. This is due to the low economic value that caiman meat holds. It only benefits them to kill large caiman; however, they are not willing to venture into inaccessible lakes where M.niger tends to breed (Da Silveira, R. Thorbjarnarson, J, 1999). 5 A cause of hunting is that loggers, miners and workers from petroleum companies tend not to be provided with food from external sources. Instead, they are encouraged to hunt in the forest for bush meat. Also, the translocation of some communities into more rural, uninhabited areas, has led to an increase in hunting that has not previously existed. This was the case for the Transmigration Program in Indonesia, and also the PolaAmazonia Program in Brazil (Bodmer, Robinson, 1999) 1.5 Ranched and Captive-Bred Crocodilians Sustainable use is a term popular with all biologists, including crocodilian specialists. It is defined by the IUCN as the ‘use of wildlife associated with a process aimed at ensuring the use can continue indefinitely and that its impacts are maintained within prescribed limits’ (IUCN SSC 2008). The meaning of the word ‘use’ in this context refers to the species being used as part of a management program to benefit human populations; it refers to keeping a population in a ranch or farm for the exploitation of the caimans’ meat and hides. The main difference between ranched and captive bred crocodilians is those captive-bred are born into captivity, however, those who have been ranched have been taken out of the wild at a vulnerable age that can be considered to be at risk from mortality: such as eggs or hatchlings. CITES has its own definition of ‘captive-bred’ (in CITES Resolution Conf. 10.16 (Rev.)), which states that the term refers to specimens ‘born or otherwise produced in a controlled environment’ if: i) The parents were in a controlled environment at the time of development of the offspring. ii) The breeding stock was established legally and in a manner approved by CITES. It must also be maintained without the introduction of specimens from the wild (with certain exceptions) and must have produced offspring of at least second generation in a controlled environment or be managed in a manner that has been demonstrated to be capable of doing so (MacGregor, 2006). Of course, any form of captivity comes with animal welfare issues, Arthur Lindley, previous head of the RSPCA’s wildlife department, carried out research into reducing mortality in crocodile ranches in Zimbabwe. He proposed the introduction of drainpipes into the enclosures in order to reduce stress by providing the crocodiles with a form of refuge from any human presence. Instead of the fleeing animals hitting the solid sides of the enclosure, causing them harm and additional stress, they would be able to use the drainpipes as a shelter to conceal themselves (Harrop, 2010). Animal welfare issues such as this need to be taken into account when considering the positive and negative aspects of ranching and captive breeding programs. Ranches and farms needs to reach a certain standard of care are accepted by CITES 6 before they can be registered with the CITES Secretariat, which then allows them to trade legally under CITES. This approval may be withdrawn if they fail to comply with the required conditions. A study in 1998 stated that in South America, investors were encouraging the establishment of caiman farms and ranches to allow the legal exportation of skins. If the farm were to be run properly, it would only involve taking one initial batch of live adults from the wild, and then breeding these to produce increased individuals to sell as hides and meats. For ranches, it is wild eggs or hatchlings that are taken from the wild to start the captive population, the young are raised until they are one metre long, when they would be harvested for their hides. The continuing resource for ranches is the wild; hence, ranching is often viewed as an incentive for conserving high populations in the wild, to ensure there is always an accessible population for ranch owners. However, this is often far from the truth, for example, captive breeding operations in Colombia are obliged, legally, to provide individuals to restock wild populations, but are nonetheless deemed to be providing little assistance to wild caiman conservation (Larriera et al, 2004). Also, some so-called ranches are just wild areas fenced off by a private owner and subsequently classed as a farm or ranch, the caiman sold from these establishments are those that were unfortunate enough to have previously inhabited the area. It is difficult to determine if hides or meat being exported have been farmed, as it claims to be, or has actually been illegally caught from the wild. Also, a continuing decline in ranch size puts owners under pressure to increase their maximum revenue per area, which will involve taking more caiman from the wild to be killed and exported. There are a substantial number of further problems for ranching other than captive-breeding: restricted access to wild populations, and the cost of harvesting wild populations. As a result, captive breeding is the major form of production for skins in a number of countries, including, Colombia (Caiman crocodilus), Mexico (Crocodilus moreletii) and Thailand (Crocodilus siamensis) (CITES trade data, 1999). There are a number of dangers to wild populations when a country has a commercially successful captivebred operation in place. Firstly, interest in maintaining the population’s habitat tends to be lower, and they become inclined to transform the habitat for a more constructive personal use. Secondly, local farmers and landowners are unlikely to be as tolerant to crocodilians living in close proximity as they pose risks to both human populations and livestock. This is where ranching holds a prominent advantage, as one of the chief prerequisites for approval of a ranching scheme under CITES is that it should ‘be primarily beneficial to the conservation of the local population (i.e. where applicable, contribute to its increase in the wild or promote protection of the species’ habitat while maintaining a stable population)’ (CITES Resolution Conf. 11.16). This resolution ensures that all ranching programs have some element of 7 conserving crocodilian populations, and that ranches are not viewed as equivalent to captive breeding programs. By having a coordinated system that allows for legal exportation, the authorities may be able to regulate exports through borders more efficiently. However, if South Americans have been receiving a reasonable income through an illegal lifestyle, there is a slim chance they will change their habits because of the law. Also, many of the countries caiman ranches and farms are present in, have a poor history at wildlife law enforcement; therefore, it is unlikely the ranches and farms will be legitimate. Furthermore, the revenue acquired from ranching will not be put back into conservation; more likely it will go towards the production of more ranches and farms. No funds will be put towards preserving the caiman’s natural habitat, and therefore the populations left in the wild will not be able to maintain themselves as they have suffered habitat loss and degradation through the infrastructure required for the farms and ranches. In principle, a farm can work towards restoring a broken population through hosting a breeding program; this has proved successful in India where they have farmed the saltwater (Crocodilus porosus) and mugger crocodiles (Crocodilus palustris) and put funds into restoring their original habitats to ensure a successful, future reintroduction (Ron et al, 1998). 1.6 Degradation of land Habitat destruction is present in both obvious and more subtle forms. Deforestation, drainage, infilling, pollution, and conversion to agricultural use are some of the main causes of habitat destruction (IUCNSSC, 2008). When the latter was the case in the Philippines, it caused the virtual disappearance of two species: the Philippine crocodile (Crocodilus mindorensis) and saltwater crocodile (Crocodilus porosus). Freshwater marshes are often used by the saltwater crocodile for nesting. Although there are still plenty of freshwater marsh areas available, they are now being used for agriculture, so the crocodiles that nest there are being killed by the farmers (IUCN-SSC, 2008). The erection of dams can also be a cause for concern as a form of habitat destruction, and dams have varying levels of success in relation to populations of crocodiles. Initially, the suitable habitats within the area of the dam, the marshy, freshwater habitats are destroyed, and populations of residing crocodiles will decline. These previously successful habitats are replaced with simple, bare, shorelines. The water impoundments created from the dams have then proved successful in supporting the local crocodile populations. However, due to an increased demand for water, for either agriculture or hydroelectricity, the water levels fluctuate regularly and the reproductive success of some populations has decreased. This has occurred in impoundments in Honduras, India and Zimbabwe (IUCN-SSC, 2008). 8 2.0 Species studied There are five species of caiman found in Peru: American crocodile (Crocodilus acutus), common caiman (Caiman crocodilus), black caiman (Melanosuchus Niger), smooth fronted caiman (Paleosuchus trigonatus), and dwarf caiman (Paleosuchus palpebrosus). Out of these, there are three found in the Pacaya-Samiria Reserve: common caiman (Caiman crocodilus), black caiman (Melanosuchus Niger) and smooth fronted caiman (Paleosuchus trigonatus). However, during this census study, no Paleosuchus trigonatus were observed, and therefore they are not included in the remainder of this paper. 2.1 Caiman crocodilus, (Common or Spectacled caiman) There are three subspecies generally recognised of C.crocodilus, C. c. crocodilus, C. c. apaporiensis, and C. c. fuscus. There is also a fourth, but this is now classed as a separate species, Caiman crocodilus yacare. C.crocodilus is a generalist species and is extremely adaptable in terms of habitat choice being both a terrestrial and fresh water habitat species. They are found in most lowland river and wetland habitats throughout the species’ range. C.crocodilus has the widest distribution of all the Alligatoridae family; its native geographic range includes many Central and South American countries including: Brazil, Colombia, Ecuador, French Guiana, Guyana, Honduras, Mexico, Panama, Peru, and Venezuela. The species has also been introduced to Cuba, Puerto Rico, and the United States. Below are C.crocodilus’ IUCN red list classifications from 1986 to its current status: 1986- Threatened 1988- Threatened 1996- Lower Risk/Least concern As with all crocodilians, in 1975, C.crocodilus was listed on Appendix; however, as of 1977, CITES lists all populations of C.crocodilus on Appendix II. This species has benefited from the commercial utilisation of other species such as, Crocodilus acutus, C.intermedius, and M.niger. As an opportunistic species, it has been able to dominate areas previously occupied by those species which have declined due to hunting pressures. However, this species has also suffered from hunting pressures when other, sympatric species levels declined, and has been extrapolated from its natural habitat for use in the pet trade, but currently population levels are in good condition. 9 2.2 Melanosuchus Niger (Black caiman) This species is present throughout the Amazon basin and its distribution includes: Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Peru and Venezuela. The M.niger is a specialist species in relation to habitat choice, and can be found in freshwater habitats, such as slow moving rivers and oxbow lakes. The M.niger has been heavily exploited since the 1930’s after stocks of other South American crocodiles, (Crocodilus acutus and Crocodilus intermedius) were becoming very low (Da Silveira, Thorbjarnarson 1998). As with C.crocodilus, M.niger was listed on Appendix I with the introduction of CITES in 1975, it was not until 1997 that ranched populations of the species in Ecuador were down listed to Appendix II. Further to this, in 2007, this was broadened to include Brazilian populations; these are both now subject to a zero annual quota until an annual export quota has been approved by the CITES Secretariat and the IUCN/SSC Crocodile Specialist Group (UNEP-WCMC 2010). Below are the IUCN red list classifications for m.niger: 1988- Endangered 1990- Endangered 1994- Vulnerable 1996- Endangered 1999- Lower Risk/Conservation Dependent 2000- Lower Risk/Conservation Dependent 3.0 Study site The study was carried out for 17 days over the period 30th May 2009 - 15th June 2009. The study took place at Pacaya-Samiria National Reserve, along the Samiria River, one of the three major tributaries whose basins drain the Pacaya-Samiria. We were based at PV3, (-5.0533°W, -74.5261°S) in the lightly hunted zone of the reserve, (which is in comparison to the adjacent areas being classed as moderately and persistently hunted zones). Pacaya-Samiria was first designated as a protected area in 1940, and in 1972 it was classified as a National Reserve, then in 1982, the area was expanded to its current size of 2,080,000 ha (Bodmer et al, 2009). 10 Figure 1: Map of the Pacaya-Samiria National Reserve, PV3 indicated by the black circle (Bodmer, R. 2009). The reserve is located in the Loreta Region and is surrounded by two rivers, the Marañon to the north and Ucayaly to the south, it is the largest reserve in Peru, and fourth largest in South America. There are an estimated 95,000 people living in villages or towns along the boundary of the reserve, there are no settlements within the core of the actual reserve, those indigenous communities that do live around the area are known as Cocama-cocamilla Indians (Puertas et al 2000). Presently, there are a number of projects underway to make their lifestyles sustainable. Initially, with the creation of the reserve, all communities were excluded in order to preserve it; currently though, the local communities are intensely involved with the management of the reserve (Bodmer et al, 2007). This change in treatment of the local communities has shown a reduction in hunting pressures and an increase in wildlife conservation. The change in attitudes and the natural resources the communities have been allocated, allows them to see the potential use of the reserve and the importance to conserve it for the future. The principle objective of the Pacaya-Samiria National Reserve is to conserve the representatives of the southern jungle of the Amazon. It is also important to preserve its genetic diversity, support the research that takes place there, facilitate the socioeconomic development of the surrounding towns by encouraging sustainable use of the wild fauna and flora to the surrounding towns, and also to encourage local tourism. 11 The reserve comprises a white water, nutrient rich, pH neutral, and várzea habitat. This ‘flooded forest’ habitat is constantly changing due to the movement of sediment and nutrients, as water levels rise and fall during the seasonal floods. This is when the sediment moves and creates unique characteristics, such as levees and point bars. As the area is dominated by tropical rainforests, the climate is of a high humidity and even higher heat; temperatures can rise to above 34°C. The dry season lasts through from May to October, whilst during the wet season, from December through to March, frequent and heavy rain showers occur and the area can receive up to 120 inches of rainfall per year. 4.0 Aims of the study The main aims of this study are as follows: 1) To compare the abundance of Caiman crocodilus and Melanosuchus niger in the Pacaya-Samiria National Reserve. 2) To compare the distribution of Caiman crocodilus and Melanosuchus niger in the Pacaya-Samiria National Reserve. 3) To look at size and sex ratios to determine the recovery of Melanosuchus niger. The above is being done to establish if the populations are successfully regenerating themselves in spite of past and present hunting pressures. Also, the analysis will determine if the protection provided by the Pacaya-Samiria Reserve is having a positive effect on the populations of Melanosuchus Niger and Caiman crocodilus. 5.0 Methodology 5.1 Census method The study included both a general census and a capture method for those individuals below two and a half metres. The transects were split between upriver (-5.0549°, -74.5260°) and downriver (-5.0532°, 74.5264°) from our base at the research vessels, and a channel (-5.0544°, -74.5261°) to an oxbow lake. The two river transects consisted of upriver and downriver, and in addition to this, each transect was divided into right hand side and left hand side, but this has not been made apparent in the presentation of the results. A Global Positioning System (GPS) was used to accurately measure the length of the transects, and also to allow us to repeatedly cover the same, identical transects. The distance travelled on the river totalled 12 95.7 kilometres; and on the channel 80 kilometres. The table below shows how far was travelled on each of the six transects. We had started with five kilometre transects, however, due to the high water levels; we were not observing as many caiman as necessary for the study. To overcome this, we attempted to travel for 15 kilometres per transect. Transect Total Surveyed (km) Upriver 1 24.7 Upriver 2 31 Downriver 1 15 Downriver 2 15 Channel 1 43 Channel 2 37 Average length travelled on each transect (km) 1. Upriver right 8.23 2. Upriver left 12.5 3. Downriver right 5 4. Downriver right 5 5. Channel right 10.75 6. Channel left 12.3 Table 2: Total area surveyed and average length surveyed on each transect. The census took place after nightfall, using a motorised boat with a 15 horsepower engine. The censuses started at roughly 19.30 with a research team of five, including three biologists, one practiced caiman catcher and a driver for the boat. The same team of researchers were used throughout the census to provide consistency with the observations and avoid any biases from differing capture ability. The transects took between 90 and 240 minutes to complete depending on distance travelled, weather conditions and number of individuals captured. The methodology used of nocturnal spotlight, is the most commonly used method of sampling crocodilians. The individuals were spotted by their eye shine, because when a 12 volt spotlight is flashed at their eyes it produces a reflection off the part of the caiman’s membrane, (tapetum lucidum). The boat tended to travel at approximately 20 metres from the shoreline, at a speed of below one kilometre per hour. 13 When an individual was spotted but was too wary to be caught, the visible crown of the head was used to note the species and the length of the head was used to estimate the total length of the reptile. Other morphological characteristics of the caiman’s head such as, the skull shape, and prominence of ridges, also acted as an estimate for the individual’s length, and consequently its age (Magnusson, 1983). Once all measurements had been taken and recorded, the caiman were released back into the water immediately. 5.2 Captures To capture suitably sized individuals an iron-wire noose attached to a 2 metre wooden pole was used; some of the smaller juveniles were able to be caught by hand. Once captured, the caiman’s limbs and jaws would be tied using heavy duty string, and the jaws secured shut, therefore securing the individual. The data recorded included the species, weight, sex, and type of vegetation the individual was found in. The measurements we then took were: total length, snout to vent, total head, and muzzle to eye, using a tape measure. Figure 2: Capturing a C.crocodilus juvenile. Figure 3: A secured juvenile C.crocodilus. 14 5.4 Species identification M.niger has dark colourings with yellow markings across the body which fade with age. The lower jaw is a pale colour with large, dark spots. M.niger is the largest in the family Alligatoridae; they can reach a length of four metres, a lot of their length is in the tail. Their structure is relatively different from other caiman species: particularly in the shape of the skull, with larger eyes and a narrow snout (Britton, 2009). Figure 4: Melanosuchus Niger C.crocodilus juveniles are a yellow colour with black markings of bands and spots on the tail and body. As the individual matures, it loses its markings and becomes a brown/olive green colour. It gets its common, ‘spectacled’ name from the intra-orbital bridge of bone that lends a bespectacled appearance. C.crocodilus is a small to medium sized crocodilian. The females can reach 1.4 metres and the males will reach a ................................................ Figure 5:Caiman Crocodilus ....larger size of 2 to 2.5 metres (Britton, 2009). 5.3 Sexing an individual Determining the sex of an individual can be difficult visually; size is the only indication, as males tend to be larger than females, but even this can portray the incorrect sex. In order to sex an individual correctly, the penis or clitoris must be felt or seen. To do this on a mature individual, a clean finger can be inserted into the cloaca to feel for the organ. However, in juveniles, blunt forceps need to be used to spread the vent so you can view the inside of the cloaca; there you will be able to see the cliteropenis, so called due 15 to the female’s clitoris and male’s penis having a similar appearance in hatchlings. Due to this similar appearance, experience is necessary to be able to sex a young juvenile. Figure 6: Sexing an adult. Figure 7: Sexing a juvenile male C. crocodilus 6.0 Statistical Analysis To calculate the analysis below Microsoft Excel was used. 6.1 Abundance The abundance of the two species on both habitats was calculated as individuals per kilometre. The equation below was used: Abundance = n/l Where n= the total number of individuals and l= the total length of the transect. Mean averages were also calculated to determine the abundance of differing size ratios. 6.2 Standard Deviation Standard deviation is a measure of variability. It is calculated from all observations within a particular sample. The following equation is used to calculate the standard deviation. 6.3 One way ANOVA 16 This is a statistical test used to compare the means of two samples and determine whether the differences between the sample means are significant. It is used as a measure of variability. This test was calculated using the level of P=0.05 during this study. 6.4 Chi-square test The most commonly used method of analysing frequencies is the chi-square test. It tests if our observed frequency data is significantly different from frequency data we could normally expect. The test calculates one single figure that can be directly compared to a critical x2 value. The chi-square test will be used for sex and size ratios in relation to habitat use and choice of transect. (Frankham, 2004) 7.0 Results The following results were composed to be able to assess the abundance and diversity of the three possible species of caiman found in the Pacaya-Samiria National Reserve; and subsequently assess the effect of trade on the populations and how successfully they are recovering. 7.1 Abundance Over a period of 17 days, a total of 87 caiman were seen. Table 2 demonstrates that only 58 of these were identified. 45 were identified as C.crocodilus, and 13 as M.niger. The final 29 were classed as ‘Eyes Only’, (when the only data recorded was the presence of an individual due to it being viewed from long distance) and the species was not identified. There were six different transects, consisting of three areas, each split into two, the left and right side. 17 Transect Number Transect Name C.Crocodilus M.niger OE 1 Upriver right 13 2 6 2 Upriver left 5 1 3 3 Downriver right 2 0 2 4 Downriver left 0 2 2 5 Channel right 15 4 7 6 Channel left 10 4 7 45 13 27 Total Table 3: Abundance of all caiman observed on the six different transects. Total Individuals Average Seen per transect Upriver 30 5 Downriver 8 4 River (Upriver + Downriver) 38 4.75 Channel 47 6.71 Total 85 5.12 Mean number of individuals seen Table 4: Abundances of all species on each transect, and mean observed and caught per transect. Figure 8: The mean abundance of all individuals observed on three different transects 2.5 2 1.5 C.crocodilus 1 M.niger 0.5 Only Eyes 0 Downriver Upriver Transect Channel Table 3 and Figure 8 (see above) both illustrate that C.crocodilus had relatively high abundance in both the upriver transect and the channel. The pattern of C.crocodilus, M.niger and ‘only eyes’ are consistent 18 throughout these two transects, with M.niger being second highest in abundance, and ‘only eyes’ the lowest. Downriver, however, the same numbers of M.niger and C.crocodilus were observed, and higher levels were seen of unidentified individuals, ‘only eyes’; but overall a much lower abundance was found. This is of course partly due it being the transect executed the least, however, that was due to the lack of caiman observed. The figures for abundance of caiman per kilometre are as follows: C.crocodilus:0.72 per km M.niger: 0.19 per km Only Eyes: 0.4 per km Total: 0.43per km In the Pacaya-Samiria Reserve during the period July to August, two months after our study was completed, there was an average of 4.57 caiman seen per kilometre. In comparison to 1980 when recorded densities were 9.02 caiman per km, Pacaya-Samiria has suffered a noteworthy loss. 7.2 Size Classes Number of individuals Figure 9: The size classes of all individuals observed in both the channel and river transect. 18 16 14 12 10 8 6 4 2 0 <60 60-119 120-179 >180 Channel C.crocodilus River Channel M.niger River Transect As illustrated in Figure 9, there was a healthy population of hatchlings and juvenile C.crocodilus observed on both transects. There were not any larger individuals of this species seen, however, if we assume this is due to C.crocodilus being a smaller species then we observed individuals from all stages of 19 the species development. There were no hatchlings or juveniles for M.niger, only sub-adults and adults. See Appendix III for size classes of all individuals observed, calculated as mean number of individuals seen per kilometer. Channel River C.crocodilus 53.9 69.75 M.niger 166.37 120.54 Table 5: The mean lengths of M.niger and C.crocodilus on both habitats. Species S.d M.niger 64.45 C.crocodilus 23.87 Table 6: The standard deviation of the size classes of the two species. Chi square was also calculated for the size classes of the two species. Yates’s correction was applied here due to there only being two categories. C.crocodilus: x2= 1.92 d.f = 1at P= 0.05 M.niger: x2=0.33 d.f = 1 at P=0.05 Both of the x2 values are below the tabulated value of 3.84, therefore there is no significant difference between size classes and the habitat the individual was found in 7.3 Habitat Figure 10: Microhabitat use of all species on the two transects 50 40 30 Channel 20 River 10 0 Between Dense Floating Forest Mixed vegetation vegetation vegetation vegetation vegatation 20 Open water As Figure 11 demonstrates, floating vegetation was the microhabitat individuals were found most often in, which could be due to the large amount of water lettuce (Pistia stratiotes) that had drifted through the channel from the oxbow lake due to the high waters. See Appendix II for the microhabitat use between both species on the river and channel transects. ANOVA: Between the two different habitats, the river and channel. There was no significant difference in abundances between the two habitats of the river and channel for C.crocodilus and M.niger. River: F=4.38 df = 20 P=>0.05 Channel: The null hypothesis of ‘There is no significant difference between species abundance in the river and channel habitats’, was automatically accepted without calculating an F value. This was due to the ‘Within samples variance’ being larger than the ‘Between samples variance’. P=>0.05. Chi-square test: Between the two different habitats, the river and channel. The chi-square test was calculated to Yates’s correction; this was used as there were only two categories, the river and channel habitat. The following equation was used: 2x2 = (X = ((O-E)-0.5)2 / E). C.crocodilus: X= 0.36 d.f=1 at P=0.05 M.niger: X=0.7 d.f=1 at P=0.05 Both of these figures show that the observed frequencies agree with the expected frequencies at the two habitats. Thus there is no significant difference between the observed and expected frequencies of microhabitat use. 7.4 Sex Ratios The chi-square test was carried out on sex ratios. However, due to the lack of data of caiman sexes from May-June 2009, the data from Pacaya-Samiria July-August 2009 (Courtesy of Earthwatch) was combined to create a larger, more practical, quantity of data. M.niger. x2 = 1.05, df=1, P=0.31. C.crocodilus. x2 = 0.08, df=1, P=0.77. 21 The tabulated value in the x2 table was 3.84. Therefore, M.niger and C.crocodilus show no significant difference in the ratios of the sexes caught within their habitat. Number of individuals 7.5 Lunar Phases Figure 11: A Comparison of Observations, Captures and Lunar Phases of all species. 30 25 20 15 10 5 0 Moon not visible 1/2 Moon 3/4 Moon Full Moon Species and activity Figure 10 demonstrates how the highest number of observations or catches happened when the moon was not visible. There was not necessarily the lowest number when there was a full moon, but when there was any evidence of the moon, the numbers dropped dramatically. 8.0 Discussion Throughout the discussion, postulations and conclusions will be made concerning the current state of the caiman population in Pacaya-Samiria National Reserve, and thereafter propositions will suggest possible future management systems that may be of benefit to the recovering populations and also to control the consequences trade has on these populations. 8.1 Size and sex ratios There was a high number of C.crocodilus under 60cm and 60-119cm observed. This is evidence of many juveniles being present and therefore recruitment to the breeding population, which is promising for the populations of C.crocodilus in Pacaya-Samiria. However, there are not as promising figures for M.niger. Although this is the larger species of the two, there should still be some smaller juveniles observed in the 22 area as evidence for recruitment to the breeding population. M.niger grow at a rate of 30-35cm per year when young. Using this data, and the estimation that caiman hatch at a length of 15-20cm, one can assume that the previous year’s hatchlings would have been <45 cm during May-June. C.crocodilus were the only species we observed with individuals of this size, there were 15 individuals with a total length of <45cm; in contrast, there were no individuals of M.niger observed at this size. We saw very few C.crocodilus over the length of 120cm, which can be explained by the smaller size of the species, with the females only reaching 140cm. The data collected from Pacaya-Samiria during July and August 2009 shows similar results. There was only one individual of M.niger at a total length of 45.7 cm. However, during the same census period, there were 24 M.niger at larger, adequate breeding lengths, showing a possible strong breeding population. There were four individuals of a similar length observed during May-June, and this figure equals approximately one third of all the M.niger observed. Data from Lago Preto Conservation Concession, June-July 2009, offers similar results with four out of the five M.niger seen being of breeding length (Operation Wallacea, 2009). There have been various ideas postulated about the effect of hunting on crocodilians, these include: male based sex ratios due to a preference in hunting females (Mourao et al, 1995), decreased nest attendance by females and an altered population structure. It has also been suggested that hunters prefer individuals with a measurement of over 80cm: snout-vent length, but there is no evidence of a preferred sex (Mourao et al, 1995). 8.2 Niche overlap Melanosuchus niger and Caiman crocodilus have overlapping distributions throughout their ranges. Both species prefer smaller, quieter lakes, rivers and channels; they are both categorized as generalist carnivores and both eat terrestrial invertebrates when young, and replace these with molluscs and fish as they mature (Herron, J. 1994). It would appear from this information that the two species’ niches should majorly overlap, however, there is little evidence to determine if each species affects the others abundance and distribution. 8.3 Genetic issues Small or declining populations are more prone to extinction than those that are larger and more stable. A small population will suffer a loss of genetic diversity and experience the bottleneck effect; the result of this is a decline in evolutionary potential (Frankham et al, 2004). Genetic drift may also prove to be a limitation for recovering populations of caiman; smaller populations are more prone to experience genetic drift than larger ones. 23 Very little information is known on the genetic diversity or meta-population structure of these two species. This data could prove to be vital for conservation programs of wild and captive-bred populations. This information could also be used for the mitigation and prevention of fitness losses associated with the isolation and decline of the populations of the two species. A study conducted by Farias et al (2004), tested to see if M.niger and C.crocodilus were genetically similarly structured. It also investigated if they portrayed any signs of becoming genetically depleted due to overexploitation. It was discovered that genetic diversity was slightly higher in M.niger than C.crocodilus, which is unexpected due the lower population numbers that M.niger has suffered. In addition, the level of gene diversity differed depending on the habitat the individuals were found in, and if it were a white-water varzea habitat or a black-water habitat. This may be due to the low nutrient, and high acidity levels present in black-water habitats compared to the nutrient rich and near neutral pH of the white-water habitats. Irrespective of these genetic differences, both species still showed a higher level of genetic diversity than Alligator mississippiensis. This suggests that the overexploitation suffered from hunting pressures have not necessarily depleted the important, high levels of genetic diversity (Farias et al, 2004). Some of the populations observed in the study appear to be undergoing a demographic expansion in white-water habitats; this bodes well for the populations of M.niger and C.crocodilus in Pacaya-Samiria; a similar white-water habitat. Limitations: 8.4 Wariness of caiman When conducting caiman censuses, the wariness of the reptile should be taken into consideration as it could affect the results. It has been proven that size affects the wariness of caiman and it has been proposed that hunting and capturing can too (Pacheco, L. 1996). Pacheco’s study confirmed that caiman of a larger total length were more wary than shorter individuals. The suggestion that some environmental factors, for example lunar phases, may affect the wariness of caiman was not proven by his survey. Our results, however, indicated that this was the case. The study did not take place during any new moon phase, but we did suffer weather conditions that provided cloud cover so no moon could be seen and its illumination was not encountered. There were a noteworthy number of more observations and captures when the conditions did not allow for any illumination to be observed. Suggestions for this are two fold. Firstly, that caiman have increased sight abilities with the assistance of the moon’s illumination, and therefore can see the boat and human activity more so than if there was no moon, and consequently avoid the boat. Secondly, the eye-shine reflection we were looking for to spot the caiman may not be as visible when there is illumination, and so our level of observation was lower. 24 If a certain population has been subjected to a high level of hunting, the population is more likely to be wary of human activity. Also, if there is a specific area where more censuses or capturing of caiman takes place, then these populations may also be more wary than others. However, the idea has been postulated that being wary of human activity is an inherent property that comes with age (Pacheco, L. 1996). During our study period, a female C.crocodilus individual of a total length of 57.2 cm was captured on two consecutive nights. The small size of this individual agrees with Pacheco’s work in that being wary comes with age, so a juvenile is less cautious of human activity. Whatever the reason may be, it is strongly suggested that the results from the study undertaken in Pacaya-Samiria were affected by the caimans’ wariness. The population census took place alongside a study of stomach contents of the caiman. This study required definite captures and handling of the caiman. The Pacaya-Samiria Reserve is annually used for caiman censuses and stomach content studies; therefore, the caiman residing in the area have most probably grown wary of the frequent boat movement and human activity. If this study were repeated in the same area, the monitoring and census methods should be designed with the caiman’s wariness in mind. This will ensure there are as few biases and inaccuracies as possible. Caiman being wary can prove to be disadvantageous for the individual and species’ as a whole. Firstly, being wary will result in the individual having increased energy expenditure as it moves to be avoided. Secondly, the caiman will have less time for foraging and reproductive activities. This could cause a decrease in hatchings, and consequently, a lack of recruitment to the breeding population. Finally, although these high levels of wariness prove a hindrance for census studies, it implies that these caiman will also flee from and not be observed and captured by hunters; therefore, leaving a good level of large breeding individuals in the wild. 8.5 High waters The rainfall at Pacaya-Samiria prior to our arrival was exceptionally high, the geomorphology and biogeography of this region are influenced by very active fluvial dynamics, and the rivers from the Peruvian Andes and the Ecuadorian Andes swell and exceed their banks after dramatic amounts of seasonal rainfall in these watersheds (Sears, R. 2001). A study that suffered a similar restriction to this study took place during the high water season in Ecuador in 1998. They suffered reduced census data as a result of the high water levels that impeded their final results and did not provide an accurate population estimate. Santiago commented on M.niger and C.crocodilus staying in the flooded forest region during the high water season (Santiago et al, 1998). Strong evidence that the Pacaya-Samiria study was affected by the high water levels is presented through the data during July and August. There were ten times as many caiman seen per kilometre during this 25 period than May to June. This is obviously not a natural or expected population increase, and therefore, the high water levels must be responsible. 8.6 Further limitations caused from human activity During the census period there was more boat traffic and disturbance than usual. Over the 15 day period, an estimate of 120 additional motorised boat trips were carried out, there were also additional canoe trips, but it is doubtful these would have delivered the same amount of disturbance as a motor. The research vessels that acted as a base for the study ran the generator twice a day for two hours, one of these sessions started before, and ended during, the census time. The noise, heat and water disturbance emitted from this may have had an effect on the presence of caimans in the surrounding area. As the census began between only one to two kilometres from the boat, the caimans nearby were possibly deterred, and fled into the flooded forests. This could also explain our lack of observations downriver, as any pollution would have been carried this way. As the two research vessels are not a permanent fixture at PV3, the pollution caused by them may well have had an effect on the nutrient levels of the water. The waste discharged from the boat will have included substances alien to the ecosystem. During each transect, the number of people onboard the boat differed, and therefore, the noise and disturbance differed. It is assumed that the higher number of people that were present on the boat, the more aware and cautious the caiman would have been. If repeating the study, it would be interesting to record the number of people on the boat per transect and compare this to the number of caiman observed and caught, and to assess this correlation. 8.7 Hunting resilience There have been many postulations as to why C.crocodilus recovers more successfully from hunting pressure than M.niger. A study of populations of C.crocodilus in Brazil and French Guiana demonstrated that they were increasing at an order of magnitude faster than the population of M.niger at the same habitat (Farias et al, 2004). A study of C.crocodilus in the Venezuelan Guyana region in 1978 used the mark-recapture method to determine the growth rate of this species. It was estimated that caimans reach a total length of slightly less than one metre in six years; also that caimans less than two years old grow steadily throughout the year, however, the growth rate slowed during dry seasons. Two caimans in the size range of 90-120cm grew 10cm during a wet season one year, but did not grow at all during a dry season. The reasons for this are unknown. Other, similar, studies such as Ouboter and Nanhoe 1984, have suggested a larger growth rate; this has been attributed to food availability in different regions (Gorzula; Slejas 1984). 26 The size of the individual at sexual maturity has been proven to have a considerable effect on the species’ resilience to hunting. Female M.niger’s tend to first breed when they reach two metres in length. C.crocodilus, however, first breed at the smaller length of 130cm, therefore, they do not take as long to reach the age of sexual maturity and can breed and add to the population at an earlier age (Brazaitis 1974). A population of C.crocodilus hunted during a one year time period would require between just eight and twelve months before recruitment to the breeding population occurred. M.niger, however, would require approximately three years before it achieved the same recruitment levels. This is an issue as the species is unlikely to receive a three year break from being hunted. Therefore, C.crocodilus populations are able to maintain a stable population throughout the hunting season when M.niger cannot. In a study undertaken by Hebelo and Magnusson, a mean of 50% of two samples of female C.crocodilus investigated were large enough to have bred at least once before being caught. Whereas, two samples of M.niger showed that the mean number of those large enough to have bred once was only 28.5%. Individuals of M.niger being hunted are not given enough time to act as part of the breeding population, and add to the population as a whole, unlike C.crocodilus. Moreover, it has been discovered that nearly 80% of the M.niger that were caught for meat had reached the snout-vent length expected for sexual maturity (Da Silveira, R. Thorbjarnarson, J, 1999). As a result, hunting for meat can be considered more sustainable than hunting for hides. 8.8 The role of caiman in the ecosystem Paine first introduced the concept of keystone species in 1969 (Paine, 1969). There are two main factors in characterising keystone species; firstly, their presence is crucial in maintaining the organisation and diversity of their surrounding ecosystem; and secondly, the species need to be vitally important in relation to the rest of the ecosystem (Mills, Soule, 1983). It has been postulated that crocodilians could act as keystone species due to the positive effects they have on their surrounding environment through maintaining the ecosystem structure and its activities (King, 1998). The American Alligator (Alligator mississippiensis) already stands as a keystone species for the Florida everglades. The levels of mercury found in the alligators body has been considered as an indicator for the state of the ecosystem; the higher the level of mercury contamination in their bodies, the more pollution that is being exposed to the ecosystem through, amongst other actions, the burning of peat, fossil fuels and also mining and landfill sites (Heaton-Jones et al, 1997). Although no research has been carried out to see if there are similar results for caiman, they could prove to be an invaluable indicator species for the rest of the ecosystem. The additional contributions caiman make to the nutrient levels of the ecosystem and fish stocks is necessary to maintain so as not to cause any secondary effects. Caiman are also implicated in maintaining 27 the ecosystem’s structure and function through activities such as selective predation on fish species and also maintenance of wet refugia during droughts (King 1988). A hypothesis from Fittkau suggested that a decrease in populations of caiman will lead to a change in the original composition of an ecosystem (Fittkau, 1970). This indicates a strong association between the presence of caiman and fish. This is especially prominent during high waters, as fish from the surrounding oxbow lakes and channels may migrate into the main river to breed. Considering their place as top predators in the food chain, the caiman will follow the fish to where they migrate to feed, leading to an increase in the biomass of the ecosystem, which, as a result of their metabolism, brings allochthonous nutrients into the cycles of these nutrientpoor lakes (Fittkau, 1970). This increase will result in a higher level of nutrients in the river’s ecosystem; therefore, removing too considerable a population of caiman could cause a significant change in the rivers ecosystem and nutrient levels. There is some evidence to show that large scale killing of caimans may have disrupted the nutrient levels in clear and black river waters. This would have been through the caiman’s vital role in recycling already scarce nutrients. They also increase primary production by fertilising the water with their faeces (Smith 1981). Caiman’s position as a vital food source for rural communities is also one not to be overlooked. Through the lack of caiman as food, communities and hunters will only turn to other species, perhaps more at risk, and not as stable in maintaining their own populations. 8.9 Future Recommendations for Conservation Management Crocodilians have existed since their evolution from reptiles some 320 million years ago. Their successful existence so far is due to the evolution of its egg and the development of internal fertilisation. Through a number of evolutionary factors, crocodilians were able to occupy a new niche, they reduced the amount they relied on water bodies and began to dominate semi terrestrial habitats. It seems unjust that the human population is able to threaten such a long lived species in order to fulfil their materialistic requests for luxury items. Crocodilians need to be considered as living exhibits of natural history, not a resource for humans to exploit as they wish. Increasing conservation efforts are one of the few ways this will be achieved. The protection and management of species present in countries with tropical forests frequently lies with government agencies. An issue with this, however, is that wildlife conservation is rarely a priority for these agencies, with socio-economic issues often being at the forefront of their agendas (Bodmer, Robinson, 1999). Consequently, wildlife management is often more successful with the input of government agencies, non-governmental organizations (NGOs) and scientific institutions. This is the current situation for Pacaya-Samiria Reserve working alongside the Wildlife Conservation Society 28 (WCS) and Durrell Institute of Conservation and Ecology (DICE). Combining these institutions, government agencies and organisations, and encouraging community-based wildlife management could result in Pacaya-Samiria exhibiting a successful and productive ecosystem. Establishing conservation programs and an annual legal quota could benefit the government through collecting user fees and taxes. PROFAUNA, (Venezuelan Fish and Wildlife Service), was a system created in 1989 to ensure that program fees were channelled directly to the wildlife authority within the government. However, it has been difficult to guarantee that the money generated from a species’ specific sustainable use program has been directed back into the conservation of that same species (Thorbjarnarson ii, 1999). The establishment of sustainable harvest programs may be the solution to ensure populations of M.niger reach their full potential once more. PROFAUNA demonstrated how sustainable programs can prove successful but also includes certain flaws. One of these was that landowners that had been allocated a legal hunting quota did not want to diminish their caiman stocks too much. To overcome this, they had illegal hunting take place in various locations and the skins caught this way were sold to the landowners who passed them off as their own. Although the same numbers of caiman were killed as would have been had the hunting been executed legally, the locations the illegally hunted caiman were taken from were either public land or poorly protected private land. These few areas were then left with low caiman numbers due to the high overharvest in such a small region. Functioning this way, their quota was filled, they made sufficient profit and their stocks were still ripe for the next season (Thorbjarnarson, Velasco, 1999). A further flaw is that landowners aim to fill their quotas as soon as possible, they do this by hunting all sizes of caiman; however, they will receive a higher profit if they are able to sell larger skins. So, if these larger skins become available before the allocated time ends, they replace the smaller skins with the more valuable, larger skins. The consequences of this are that an excess number of caiman being killed, but the correct and legal number being exported (Thorbjarnarson, Velasco, 1999). Sustainable yield programs seem to be a likely and productive solution to provide the market with its demands whilst being able to keep the supply at a sustainable level. A lot of the existing programs require an annual cropping of wild populations. This may be a promising answer for C.crocodilus due to its high level of reproductive success (Britton 2009). A surplus male population of M.niger could potentially be harvested sustainably, with little impact on the reproductive potential of the population due to their presumed polygynous mating system. By taking only adult, male crocodilians, there will be less effect on the population than if a majority of adult females were taken. If this is indeed the case, then hunters and traders will still be able to obtain an income whilst not affecting the populations of caiman. However, due to the difficulty in sexing caiman visually, there would be risks of inexperienced hunters taking 29 individuals that are critical to the reproductive population, potentially having detrimental effects to the population. Input from CITES in order to try and protect the species and regulate hunting and exports was put forward in resolution 8.14 as the CITES Universal Tagging System for the identification of crocodilian skins (see Resolution Conf. 11.12, Universal tagging system for the identification of crocodilian skins). This universal tagging system is to identify raw and processed hides, both whole and partial. The information on these tags includes a unique identification number; this will be identical to that on an export permit used to allow the hides to cross borders. All the evidence provided since this introduction points to a significant decrease in the illegal caiman trade over the past decade (De Klemm, 1993, CITES 2002). However, there are a number of flaws with this system. In Venezuela, for example, tanners were allowed to remove the tags so they did not interfere with the tanning process; but this gave the tanners a chance to cut up the hides and export them in smaller pieces which are not controlled. The quota that had been permitted to the hunter was then not filled, so additional, further hides were exported in their place (Thorbjarnarson, Velasco, 1999). Therefore, more caiman were killed from that particular harvest than should have been. More recently, decision 10.78 of the tenth meeting of the Conference of the Parties states that in cooperation with the Secretariat and the IUCN/SSC Crocodile Specialist Group, Resolutions Conf. 6.17 and Conf. 9.22 shall be reviewed, and further proposals shall be made for consideration at the 11th meeting of the Conference of the Parties regarding their consolidation and a system for tracking the use of crocodilian skin tags (Wijnsteckers, 2000). It is important to ensure that the populations of caiman are kept at a healthy and sustainable level, not only for the sake of the species per say, but also to protect levels of fish stocks. Studies have taken place to ascertain the risk that lifting the ban on caiman hunting may have on fish stocks residing in the same areas. Lifting the hunting ban on caiman in Amazonia will provide short term benefits to commercial fisheries but alongside this there will be detrimental affects to local subsistence fishermen. These local subsistence fishermen were previously rubber tappers who lived along the river, the collapse of rubber prices led them to move to more urban areas where they took fishing up as their source of income. However, commercial fisheries tend to enter similar areas to them and their larger and more effective nets take more fish stocks than they are able to with their traditional methods of harpoons, bows and arrows, trident spears, and single and multiple hooks. These methods are used in shallower waters, and are, therefore, not coming into contact with caiman. Commercial fishing boats, however, use 20-50m long gill-nets in the main river channel or nearby lakes, which catches the majority of the fish which are then sold in the Manaus fish market in Brazil (Peres; Carkeek, 1993). Both M.niger and C.crocodilus have 30 been known to attack gill nets as an easy source of food; the nets are often left damaged and irreparable, causing a loss of profits to the commercial fishermen. Local opinion suggests that this behaviour exhibited by the caiman can be enough to discourage commercial fisheries from exploiting the area, resulting in more productivity for the subsistence fishermen, and less risk of over-exploiting certain fish species. If the ban on hunting were to be lifted then the commercial fisheries would most likely re-enter the area, as they would be permitted to kill any caiman spotted, and also leave the subsistence fishermen with lower stocks. 9.0 Further study To determine the effect of the high water level on the results of the census, data regarding the decrease of the water levels whilst we were conducting the census could be collected and compared to how many individuals were being observed as the time progressed. However, this may be more effective and show a greater correlation if the census were to take place over a longer time period. Another aspect of the study which could be carried out when there are lower water levels is nearest neighbour relationships. This would assist in the evidence for niche overlap of the two species to establish if they share similar microhabitats and reside in areas of similar water depths. This is thought not to be the case as M.niger is the larger species, and as a result will need to be in deeper waters to conceal themselves. A study comparable to this, undertaken in an alike Peruvian lake, found that although the two species were in fact in the same areas, they tended to be segregated spatially. C.crocodilus were prone to settle at the ends of the lake, whereas M.niger were more inclined to dwell in the centre of the lake, it would be assumed that this is the deepest part of the lake, and as mentioned previously, M.niger would require the deeper waters. This difference may be due to one of the species purposely excluding the other from a particular microhabitat or just a coincidental differing preference of microhabitats (Herron, J. 1984). Herron’s study, in 1994, postulated Magnusson’s idea that different head shapes have affected the foraging styles of the two species (Herron, J. 1984). If this were the case, it would explain the differences in preference of microhabitat, due to diverse terrains present in the varied habitats, and the ability to forage for different types of food. For this information to be correct, further study should be instigated into the foraging strategies of the two species and the biomechanics of crocodilian movements and feeding. A beneficial addition to this study would be to use stomach content data, which would allow models such as the Lotka Volterra or Schoener’s method to be applied. The calculations of these would illustrate how successfully the two species survive together in similar habitats. It would determine if there is an overlap 31 of competition for food, and also how the two species use the habitats and microhabitats to coincide productively with each other. 32 Literature Cited Bodmer, R; Robinson, J. 1999. Towards Wildlife Management in Tropical Forests. Journal of Wildlife Management. Vol. 63, (1), pp.1-13. Bodmer, R; Fang, T and Puertas,P. 2007. Wildlife populations in the Pacaya-Samiria National Reserve, Peru. Report for WCS. DICE. Bodmer, R; Fang, T and Puertas, P. 2008. Wildlife Conservation in the Samiria Basin of the PacayaSamiria National Reserve, Peru. DICE WCS. Bodmer, R; Fang, T and Puertas, P. 2008. Amazon Riverboat Exploration: Wildlife Conservation at the Lago Preto Conservation Concession, Yavari River, Peru. DICE WCS. Bodmer, R. 2009. Personal communication. Data CD. Brazaitis, P. 1974. The Identification of living crocodilians. Zoologica, Vol. 58, (3/4) pp. 59-97. Brazaitis, P; Wantanabe, M; Amato,G. 1998. The Caiman Trade. Scientific American Inc. UNEP-WCMC. UNEP-WCMC Species Database: CITES-Listed Species. Accessed 28 March, 2010 CITES. 2000. Eleventh meeting of the conference of the parties Gigiri (Kenya). CITES Doc 11.51. CITES. 2002. Twelfth meeting of the Conference of the Parties. (Chile). CoP12 Doc. 54.2. CITES, 2007. Fourteen meeting of the Conference of the Parties. The Hague (The Netherlands) 3-15 June 2007. Trade in some crocodilian species. CoP14 Doc. 43. CITES trade data, 1999. Supplemented by information provided by the CSG. Estimated number of crocodilian skins supplied to the industry by method of production (includes caiman production), 197799. Da Silveira, R. & Thorbjarnarson, J. 1999, "Conservation implications of commercial hunting of black and spectacled caiman in the Mamiraua Sustainable Development Reserve, Brazil", Biological Conservation, vol. 88, pp. 103-109. De Klemm, C. 1993. Guidelines for legislation to implement CITES. IUCN Environmental Law Centre. 33 De Klemm, C; Navid, D. 1984. Crocodilians and the law as cited in Crocodiles: their ecology, management and conservation.pp.80. IUCN. Farias, I.P; Hrbek, T; Monjelo, L.A; Silveria, R; de Thoisy, B; Thorbjarnarson, J. 2004. Genetic Diversity and population structure of Amazonian crocodilians. Animal Conservation. Vol.7, pp.265-272. E.-J. Fittkau. 1970. Role of Caimans in the Nutrient Regime of Mouth-Lakes of Amazon Affluents (An Hypothesis) Biotropica, Vol. 2, No. 2. pp. 138-142 Fowler, J; Cohen, L; Jarvis, P. 2008. Practical Statistics for Field Biology. Second Edition. Wiley. Frankham, R; Ballou, J; Briscoe, D. 2004. A primer of conservation genetics. Cambridge University Press. Gorzula, S; Seljas, A. 1984.The Common Caiman as cited in Crocodiles: their ecology, management and conservation. pp.44, IUCN. Harrop, S. 2010. Information received through personal communication. [Conversation]. March 26th 2010. Terrell G. Heaton-Jones, Bruce L. Homer, D. L. Heaton-Jones and Stephen F. Sundlof. Mercury Distribution in American Alligators (Alligator mississippiensis) in Florida. Journal of Zoo and Wildlife Medicine, Vol. 28, No. 1, Pharmacology and Toxicology (Mar., 1997), pp. 62-70 Henrique Rebelo, G. & Magnusson, W. 1983, "An Analysis of the Effect of Hunting on Caiman crocodilus and Melanosuchus niger Based on the Sizes of Confiscated skins", Biological Conservation, vol. 26, pp. 95-104. Herron, J. 1994. Body Size, Spatial Distribution, and Microhabitat Use in the Caimans, Melanosuchus niger and Caiman crocodilus, in a Peruvian Lake. Journal of Herpetology. Vol. 28, No. 4, pp. 508-513. Jelden, D. J.P. Ross, J.Thorbjarnarson, TRAFFIC South America, G. Webb 2007. Transfer of the Black Caiman Melanosuchus niger population of Brazil from Appendix II to Appendix II. CoP 14 Prop. 13 34 King, F.W. 1988. Crocodiles: Keystone wetland species. In: Wildlife in the Everglades and Latin America wetlands. Abstracts of the Proceedings of the first Everglades Nat. Park Symposium, Miami 1985. Dalyrymple G.H., W.F. Loftus and F.S Bernardino. pp. 18-19. Larriera, A., Webb, G., Velasco, A.B., Rodriguez, M. and Ortiz, B. (2004). Mission to Colombia. IUCNSSC Crocodile Specialist Group. pp 30. Luxmoore, R.A. (Ed.) 1992. Directory of Crocodilian Farming Operations. 2nd edition. IUCN, Gland, Switzerland and Cambridge, UK. 350 pp. Luxmoore, R.A., Barzdo, J.G., Broad, S.R. and Jones, D.A. 1985. Directory of Crocodilian Farming Operations. International Union for Conservation of Nature and Natural Resources, Gland, Switzerland and Cambridge, UK, and CITES Secretariat, Lausanne, Switzerland. pp. 204 . MacGregor J. 2002. International Trade in Crocodilian Skins: review and analysis of the trade and industry dynamics for market-based conservation. IUCN/SSC. MacGregor, J. 2006. The Call of the Wild: Captive Crocodilian Production and the Shaping of Conservation Incentives. TRAFFIC International, Cambridge, UK. Magnusson, W.E. 1982. Biological aspects of the conservation of Amazonian crocodilians. In: Crocodiles. Proceedings of the 5th Working Meeting of the IUCN/SSC Crocodile Specialist Group, Gainesville, Florida. 108 - 116. IUCN, Gland, Switzerland. Magnusson, W.E. 1983. Size Estimates for Crocodilians. Journal of Herpetology. Vol 17, no.1, pp86-88. Mills, S. Soule, M. 1993. The keystone-species concept in ecology and conservation. Bioscience. Vol. 43. Issue 4, pp. 219-224. Mourao, G; Campos, Z; Coutinho, M. 1996. Size Structure of Illegally Harvested and Surviving Caiman, Caiman crocodilus yacare in Pantanal, Brazil. Biological Conservation, vol. 75, pp. 261-265. Operation Wallacea. 2009. Caiman Census Data June-July 2009. Personal Communication, e-mail. 35 Pacheco, L. 1996. Wariness of Caiman Populations and its Effect on Abundance Estimates. Journal of Herpetology, Vol. 30, No. 1, pp. 123-126. Paine, R.T. 1969. A note on trophic complexity and community stability. Am. Nat. 103. pp.91-93. Peres, C.A ; Carkeek, A.M. 1993. How Caimans Protect Fish Stocks in Western Brazilian Amazonia – a case for maintaining the ban on caiman hunting. Oryx, Vol.27, No, 4, pp.225-230. Puertas. P., R. Bodmer, J. López, J. del Aguila & A. Calle. 2000. La importancia de la participación comunitaria en los planes de manejo de fauna silvestre en el nor oriente del Perú. Folia Amazónica 11(12): 159-179. Ron, S; Vallejo, A; Asanza, E. 1998. Human Influence on the Wariness of Melanosuchus niger and Caiman crocodilus in Cuyabeno, Ecuador. Journal of Herpetology, Vol. 32, No. 2, pp. 320-324. Smith, N.J.H. 1981, Caimans, Capybaras, Otters, Manatees and Man in Amazonia, Biological Conservation, Vol. 19, pp. 177-187. Statton, M.A; Dixon, J.R. 1975. Breeding Biology of the spectacled caiman, Caiman crocodilus crocodilus, in the Venezuelan Llanos. U.S Fish Wildlife Service. Wildlife Res rep. 5 pp.21 Thorbjarnarson, J. 1999.i Crocodile Tears and Skins: International Trade, Economic Constraints, and Limits to the Sustainable use of Crocodilians. Conservation Biology, vol. 13, no. 3, pp. 465-170. Thorbjarnarson, J; Velasco, A.ii 1999. Economic Incentives for Management of Venezuelan Caiman. Conservation Biology, vol. 13, no. 2, pp. 397-406. Wijnstekers, W. 2000. The Evolution of CITES. Pre-COP 11 abridged electronic edition March 2000. International Fund for Animal Welfare. pp.133. 36 Websites cited: Britton, A. 2009, January-last update, Crocodilian Species List: Caiman crocodilus. Available: http://www.flmnh.ufl.edu/cnhc/csp_ccro.htm [2010, January 10]. Britton, A. 2009, July-last update, Crocodilian Species List: Melanosuchus Niger. Available: http://www.flmnh.ufl.edu/cnhc/csp_mnig.htm [2010, January 10]. Crocodile Specialist Group 1996 2009, Caiman Crocodilus. Available: www.iucnredlist.org [2010, January 10]. Green Tracks Inc. On track with Green Tracks. 2009. http://www.greentracks.com/Newsletter/Issues/20090220-Content/Pacaya-Samiria-National-Reserve.htm [2010, January 17th] IUCN SSC Crocodile Specialist Group. 2010. Accessed 2010. http://iucncsg.org IUCN-SSC. CSG. 2009 2008, Species Accounts: Melanchosuchus Niger. Available: http://iucncsg.org/ph1/modules/Publications/action_plan1998/mnige.htm [2010, January 10th]. IUCN-SSC. CSG. 2008. Crocodile Status Survey and Conservation http://iucncsg.org/ph1/modules/Publications/action_plan1998/plan1998b.htm#Threats). Action [2010 Plan. January 20th]. UNEP WCMC, CITES Species Database [Homepage of UNEP], [Online]. Available: http://www.cites.org/eng/resources/species.html [2010, January 10th]. Sears, R. 2001. WWF Iquitos http://www.worldwildlife.org/wildworld/profiles/terrestrial/nt/nt0128_full.html. IUCN-SSC. 2008. 37 varzea. Appendix Contents Appendix 1: Raw data collected from Pacaya-Samiria National Reserve May 2009-June 2009. Appendix II: Figure 11: Microhabitat use between species on both transects Appendix III: Figure 10: Size classes of all individuals observed. 38 Appendix 1: Raw data collected from Pacaya-Samiria National Reserve May 2009-June 2009. 39 Appendix II: Figure 12: Microhabitat use between species on both transects Figure 12: Microhabitat use between species on both transects. Number of individuals seen 30 25 20 15 C.crocodilus M.niger 10 OE 5 0 Between Dense Floating Forest Mixed Open water vegetation vegetation vegetation vegetation vegatation Microhabitat Appendix III: Figure 13: Size classes of all individuals observed. Mean Number of inidviduals per km Figure 13: Size classes of all individuals observed. 0.25 0.2 0.15 C.crocodilus 0.1 M.niger 0.05 0 Hatchling <60 Juvenile 60-119 Size Class Adult >120 40