Genome Instability and Repair
advertisement
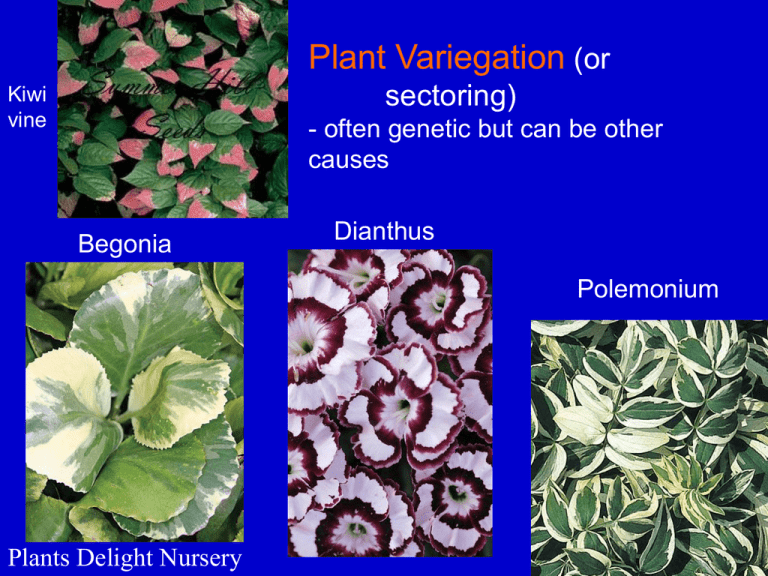
Plant Variegation (or sectoring) Kiwi vine - often genetic but can be other causes Begonia Dianthus Polemonium Plants Delight Nursery Variegation is usually nucleardetermined but sometimes cytoplasmically inherited -in this case, Mirabilis (4o’clock) , its via the chloroplast Baur, Correns Genome Instability and Repair Genome Instability: Transposable Elements • DNA elements capable of moving ("transposing") about the genome. • Discovered by Barbara McClintock, largely from cytogenetic studies in maize, but since found in most organisms. • She was studying "variegation" or sectoring in leaves and seeds. • She called them "controlling elements“ because of the myriad effects on gene expression. Barbara McClintock 1902-1992 1947 at Cold Spring Harbor 1. Nobelprize.org 1983 Nobel Prize in Physiol. & Med. - her first paper on this topic was published in 1948 2. profiles.nlm.nih.gov/LL/ Other characteristics of McClintock's "controlling elements" 1. Elevate the mutation rate. 2. Cause unstable mutations that often revert partially, sometimes giving new phenotypes. 3. Often move during meiosis and mitosis. 4. Movement (and resulting mutations) are accelerated by genome damage. Some maize phenotypes caused by transposable elements excising in somatic seed tissues. Parental plants are mutants defective in starch (endosperm phenotypes) or anthocyanin (aleurone and pericarp phenotypes) synthesis. Molecular Analysis of Transposons Transposable elements (or Transposons) were first cloned by cloning a gene from wild-type plants that they often inactivated (Federoff lab). The cloned DNA was used to isolate the gene from mutant lines. This process is also called "Transposon trapping“. Common features 1. Exist as multiple copies dispersed in the genome. 2. Insertion site of element does not have extensive homology to the transposon. 3. Contain inverted repeats at element termini. 4. A short, direct repeat of genomic DNA often flanks the transposon (i.e., integration results in a short duplication of target sequence). 5. Autonomous elements encode proteins that mobilize the element. Features unique to plant transposons: 1. Footprints: when some elements move, leave behind duplicated target sequence (footprint), which can still affect the gene (only partial restoration of gene function). 2. Two-element systems: mobility of one element depends on another. How duplications in the target site probably occur. Molecular Bases of the Myriad Effects of Transposons on Gene Expression Insertions don't necessarily inactivate genes, effects can be complex: – Insertion into a promoter can alter tissue-specific expression. – Most elements have their own promoters. – With insertions in an exon, elements are sometimes spliced out at the RNA level. - Or the inserted transposon can donate new splice sites generating new protein variants. Ac/Ds elements • Described genetically by McClintock in maize: • Ds - dissociation locus (caused chromosomal breaks), semi-autonomous element, its mobility depends on Ac • Ac - Activator, autonomous element • Cloned from the waxy (Wx) locus, which encodes UDPglucose-starch transferase • • • • Ds is derived from Ac, contains internal deletions. Both elements contain an 11-bp inverted repeat at the termini (TIR) Subterminal regions also contain repeated sequences. Both subterminal and TIRs needed for transposition, recognized by the Transposase. Structure of Ac and its Transposase Kunse & Weil, 2002 En/Spm family of Transposons • En/Spm are autonomous elements and are essentially identical. • also first cloned from Waxy locus • contain 13-bp TIR at ends • Also contain subterminal repeats • Some preference for inserting into DNA with homology to subterminal repeats. • Spm is ~ 8.5 kb and has 2 main ORFs • Alternative splicing produces 4 major transcripts and proteins (tnpA-D). • tnpA binds subterminal repeats. • tnpD binds the TIR and is probably the endonuclease. • Also a 2-element system; dSpm is defective version, contains internal deletions, and movement depends on Spm. Structure of the En/Spm Element Kunse & Weil 2002 Proposed Mechanism of Spm transposition Mu/MuDR (Mutator) • Discovered in maize; differs significantly from Ac and En/Spm families • Many copies per nucleus (autonomous and non-autonomous versions) • Contains a long TIR (~200 bp) • Transposes via a gain/loss (somatic cells) or a replicative (germline cells) mechanism. Structure of MuDR (autonomous Mu) and its promoters. • MuDrA and B expressed at high levels in dividing cells and pollen, because of transcriptional enhancers. • MURA (mudrA) is transposase & has NLS. • MURB needed for insertion in somatic cells. Mu elements moving to new sites in a cross between a Mu-active strain (or line) and a maize line lacking Mu. Retrotransposons - similar to retroviruses - move by RNA intermediate -encode a reverse transcriptase activity -can be many thousands of copies in the genome Fig. 7.34 in Buchanan et al. Retrotransposons in pea (Pisum sativum) genome Macas et al. (2007) BMC Genomics 8:427 Control of Transposons 1. Autoregulation: Some transposases are transcriptional repressors of their own promoter(s) • e.g., MurA of Mu (TpnA of Spm) 2. Transcriptional silencing: mechanism not well understood, but correlates with methylation of the promoter (similar to heterochromatin). 3. Methylation can also block binding of the Transposase (and other trans-factors) to the subterminal and TIR Biological Significance of Transposons • They provide a means for genomic change and variation, particularly in response to stress (McClintock’s "stress" hypothesis: 1983 Nobel lecture, Science 226:792) e.g., LINE retrotransposons in humans can/have caused: • Local genome instability • Genomic rearrangements, new exons, etc. (Cordaux & Batzer (2010) Nat. Rev. Genet. 10, 691-703) • or just "selfish DNA"? Or both? • No known examples of an element playing a normal role in development. Using transposons to isolate genes "Transposon tagging" • • Can be extremely powerful, isolate gene based on an interesting mutant phenotype, for example, a regulatory gene. Strategy: 1. Identify mutant caused by transposon insertion (i.e., demonstrate tight genetic linkage between mutant phenotype and presence of a copy of the transposon). 2. Fish out the gene with the inserted element from a genomic library of mutant DNA (use cloned transposon as probe). 3. Use mutant gene to fish out the wild-type gene. • Possible limitations: 1. Must use organism with known active elements. - If there are no characterized elements, use heterologous ones introduced by transformation 2. Element must integrate into the desired gene.