P & ABT III UG BT 2014 Odd sem - E

CORE PAPER- VII: PLANT AND ANIMAL BIOTECHNOLOGY
Subject description: This course presents the application of Plants in Biotechnology
Goals : To make the student to understood usage of Plant and Animal products and exploitation of them in Biotechnology.
Objectives: On successful completion of the subject, the student should have understood: Crop development, Callus culture, Biotechnological applications of plants, Animal tissue culture,
Animal products, production & improvement of them.
Unit I
Introduction to cell and tissue culture, Plant tissue culture media (composition, types and preparation), plant hormones and growth regulators in tissue culture, Preparation of suitable explants for organo genesis. Micropropagation on large scale, somatic embryogenesis, protoplast culture and somatic hybridization, Anther, pollen and ovary culture for production of haploid plants and homozygous lines.
Unit II
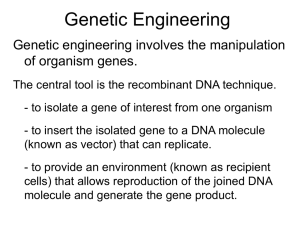
Cell culture methods for the secondary metabolite production, somaclonal variation and its significance, Cryopreservation, Gene banks for germplasm conservation. Plant transformation techniques – Mechanism of DNA transfer – Agrobacterium mediated gene transfer, general features of TI and RI plasmids and their use as vectors, role of virulence genes; design of expression vectors; use of 35S and other promoters, reporter genes.
Unit III
Animal cell cultures: Culture media – composition and preparation, Balanced salt solution and simple growth medium, chemical, physical and metabolic functions of different constituents of culture medium-Role of CO2, serum and protein-free defined media and their applications;
Culturing and maintenance of different animal cell lines (Primary and established cell lines).
Characterization of cultured cell, measurement of viability, cyto-toxicity and growth parameters.
Stem cell cultures, embryonic stem cell and their applications, cell culture based vaccines, measurement of cell death, apoptosis, scaling up animals cell cultures.
Unit IV
Transgenic animals: Method of obtaining transgenic animals using fertilized eggs and embryonic blastocyst cell, example, importance of transgenic animals – increased productivity of domestic animals, improved desired characters of domestic animals, production of recombinant gene products and proteins for pharmaceutical use. Animal models for tackling human diseases
(Gene knock out in mice models).
Unit V
Transgenic silkworms. Animal cloning: Methods of cloning in animal system – Rat, Sheep, pig; importance of cloning. Gene therapy and cell mediated therapy, RFLP maps, RAPD markers, PCR, antisense technology, terminator gene technology, DNA finger printing.
REFERENCES
1. Plant genetic engineering, Dodds J.H.
2. Plant molecule biology, Grierson and S.V. Convey
3. Molecular biotechnology, Principle and applications of recombinant DNA technology,
Bernard R Glick.
4. Plant Biotechnology-Monica Hughes.
5. Animal cell culture – a practical approach, 4th ED., Freshney. John Wiley Pub.
6. Mammalian Cell Biotechnology- A practical approach. ED Butler. Oxford UNI Press.
7. Methods in Cell Biology. VOL 57 Animal methods, ED Mather & Barnes, Academic Press .
8. Exploring Genetic mechanisms. ED Singer & Berg.
B.Sc. Biotechnology (Colleges) 2011-12 REVISED Annexure : 39A Page 12 of 25 SCAA Dt.11-05-2012
Unit I
Introduction to cell and tissue culture, Plant tissue culture media (composition, types and preparation), plant hormones and growth regulators in tissue culture, Preparation of suitable explants for organo genesis. Micropropagation on large scale, somatic embryogenesis, protoplast culture and somatic hybridization, Anther, pollen and ovary culture for production of haploid plants and homozygous lines.
Part – A
1. Who is called as the father of plant tissue culture?
Gottlieb Haberlandt
2. Emasculation
Removal of stamens or anthers to prevent self-pollination
3. Ovule
The part of the ovary of seed plants that contains the female germ cell and after fertilization becomes the seed
4. Mass selection
As practised in plant and animal breeding, the choosing of individuals for reproduction from the entire population on the basis of individual phenotypes
5. Pollen tube
A pollen tube of pollen grain grows from the stigma, through the style, and into the ovary where it enters an ovule. The sperm nuclei then pass through the pollen tube into the ovary where fertilization takes place.
6. Plant Growth Regulator
Plant hormones (also known as phytohormones) are chemicals that regulate plant growth, which, in the UK, are termed 'plant growth substances'. Plant hormones are signal molecules produced within the plant, and occur in extremely low concentrations
7. Agar agar
Agar or agar agar is a gelatinous substance derived from seaweed. Historically and in a modern context, it is chiefly used as an ingredient in desserts throughout Japan, but in the past century has found extensive use as a solid substrate to contain culture medium.
8. Pure line selection
Pure line is a self pollinated descendent of a self pollinated plant. Pure-line selection generally involves three more or less distinct steps: (1) numerous superior appearing plants are selected from a genetically variable population; (2) progenies of the individual plant selections are grown and evaluated by simple observation, frequently over a period of several years; and (3) when selection can no longer be made on the basis of observation alone, extensive trials are undertaken, involving careful measurements to determine whether the remaining selections are superior in yielding ability and other aspects of performance. Any progeny superior to an existing variety is then released as a new “pure-line” variety
9. Cross pollination
Pollination is the process by which pollen is transferred in plants, thereby enabling fertilization and sexual reproduction
10. Meiosis
Meiosis is a special type of cell division necessary for sexual reproduction. In animals, meiosis produces gametes like sperm and egg cells, while in other organisms like fungi it generates spores. Meiosis begins with one diploid cell containing two copies of each chromosome-one from the organism's mother and one from its father and produces four haploid cells containing one copy of each chromosome. Each of the resulting chromosomes in the gamete cells is a unique mixture of maternal and paternal DNA, ensuring that offspring are genetically distinct from either parent.
Part – B
1. Describe protoplast fusion.
Somatic fusion, also called protoplast fusion, is a type of genetic modification in plants by which two distinct species of plants are fused together to form a new hybrid plant with the characteristics of both, a somatic hybrid. Hybrids have been produced either between the different varieties of the same species (e.g. between non-flowering potato plants and flowering potato plants) or between two different species (e.g. between wheat triticum and rye secale to produce Triticale).
Uses of somatic fusion include making potato plants resistant to potato leaf roll disease. Through somatic fusion, the crop potato plant Solanum tuberosum – the yield of which is severely reduced by a viral disease transmitted on by the aphid vector – is fused with the wild, non-tuberbearing potato Solanum brevidens, which is resistant to the disease. The resulting hybrid has the chromosomes of both plants and is thus similar to polyploid plants.
Fused protoplast (left) with chloroplasts (from a leaf cell) and coloured vacuole (from a petal).
The somatic fusion process occurs in four steps:
1. The removal of the cell wall of one cell of each type of plant using cellulase enzyme to produce a somatic cell called a protoplast
2. The cells are then fused using electric shock (electrofusion) or chemical treatment to join the cells and fuse togethor the nuclei. The resulting fused nucleus is called heterokaryon.
3. The somatic hybrid cell then has its cell wall induced to form using hormones
4. The cells are then grown into calluses which then are further grown to plantlets and finally to a full plant, known as a somatic hybrid.
Different from the procedure for seed plants describe above, fusion of moss protoplasts can be initiated without electric shock but by the use of polyethylene glycol (PEG). Further, moss protoplasts do not need phytohormones for regeneration, and they do not form a callus. Instead, regenerating moss protoplasts behave like germinating moss spores . Of further note sodium nitrate and calcium ion at high pH can be used, although results are variable depending on the organism
Describe the characteristics of Somatic Hybridization and Cybridization 2.
1. Somatic cell fusion appears to be the only means through which two different parental genomes can be recombined among plants that cannot reproduce sexually (asexual or sterile).
2. Protoplasts of sexually sterile (haploid, triploid, and aneuploid) plants can be fused to produce fertile diploids and polyploids.
3. Somatic cell fusion overcomes sexual incompatibility barriers. In some cases somatic hybrids between two incompatible plants have also found application in industry or agriculture.
4. Somatic cell fusion is useful in the study of cytoplasmic genes and their activities and this information can be applied in plant breeding experiments.
Inter-specific and inter-generic fusion achievements
Cross
Oat
Brassica sinensis
Torrentia fourneri
Crossed with
Maize
B. oleracea
T. bailloni
Brassica oleracea
Datura innoxia
B. campestris
Atropa belladonna
Nicotiana tabacum N. glutinosa
Datura innoxia D. candida
Arabidopsis thaliana Brassica campestris
Vicia faba Petunia hybrida
3. Describe the synthetic seed preparation and its uses
Synthetic seeds or artificial seeds are the living-seed like structure derived from somatic embryoids (or, somatic embryos) in-vitro culture after encapsulation by a hydrogel. The preserved embryoids are called synthetic seeds.
Synthetic seeds or artificial seeds are the living-seed like structure derived from somatic embryoids (or, somatic embryos) in vitro culture after encapsulation by a hydrogel. The preserved embryoids are called synthetic seeds.
Father of synthetic seeds in India is Bapat who worked on sandalwood and Morus alba in1980.
Advantages
It could be preserved for long time at lower temperature
Rooting, hardening and conversion steps are waved off as these seeds can directly be sowed in the fields like natural seeds
Potential Uses
For the development of hybrid plants which have unstable genotypes or show seed sterility. In case that the seeds become sterile, the immature embryo from the seed can be rescued (called as embryo rescue) and then can be encapsulated artificially with appropriate growth medium to allow its maturation and desiccation for its germination.
Germplasm conservation
For various research and analysis purposes like studying the role of endosperm, etc
To produce large number of the clones of elite species at the cheaper cost
To supply adjuvant like plant growth regulators, pesticides, etc
Preparation of synthetic seeds:
For preparation seeds need to be encapsulated. This can be done using various water soluble hydrogels like alginate, gel rite, carboxy methyl cellulose, locust bean gum, sodium alginate with gelatin. However, alginate based encapsulation was found to be best.
Encapsulation can be done in two ways:
1. Dropping method
In this method, somatic embryos are mixed with 2% sodium alginate solution prepared in MS medium. However if the sodium alginate is purchased from an Indian based company the add 4% sodium alginate.
Then these encapsulated beads are then added as drops into a calcium nitrate solution (100mM) and then they were kept for around 20-30 minutes after which embryos are completely gelled by calcium alginate.. Then these gelled embryos are rinsed with water and then can be used as synthetic seeds.
This method has been used Redenbaugh et al. (1986) for alfalfa somatic embryos encapsulation.
However, in place of calcium nitrate, calcium chloride can also be used with by product of encapsulation as sodium chloride.
Gelling with sodium alginate is not done since sodium is toxic for cells
2. Molding
In this, embryos are mixed with temperature dependent gel like gel rite where cells get gelled as the temperature is lowered.
However, the synthetic seeds obtained by above methods cannot be used directly into the fields because:
They are very fragile
Their nutrients will be attacked by the micro flora present in the soil which will starve the embryo to death
So, to prevent above problems, one or two more coatings of alginate are given and also, certain antibiotics, insecticides, pesticides, etc are added to prevent the microbial attack on the nutrient medium.
4. Explain somaclonal variation with its advantages and disadvantages
Somaclonal variation It is the term used to describe the variation seen in plants that have been produced by plant tissue culture. Chromosomal rearrangements are an important source of this variation.
Somaclonal variation is not restricted to, but is particularly common in plants regenerated from callus. The variations can be genotypic or phenotypic, which in the latter case can be either genetic or epigenetic in origin. Typical genetic alterations are: changes in chromosome numbers
(polyploidy and aneuploidy), chromosome structure (translocations, deletions, insertions and duplications) and DNA sequence (base mutations). Typical epigenetic related events are: gene amplification and gene methylation.
If no visual, morphogenic changes are apparent, other plant screening procedures must be applied. There are both benefits and disadvantages to somaclonal variation. The phenomenon of high variability in individuals from plant cell cultures or adventitious shoots has been named somaclonal variation.
Benefits:
The major likely benefit of somaclonal variation in plant is improvement. Somaclonal variation leads to the creation of additional genetic variability. Characteristics for which somaclonal mutants can be enriched during in vitro culture includes resistance to disease pathotoxins, herbicides and tolerance to environmental or chemical stress, as well as for increased production of secondary metabolites. Micropropagation can be carried out throughout the year independent of the seasons.
Disadvantages:
A serious disadvantage of somaclonal variation occurs in operations which require clonal uniformity, as in the horticulture and forestry industries where tissue culture is employed for rapid propagation of elite genotypes. Ways of reducing somaclonal variation: Different steps can be used. It is well known that increasing numbers of subculture increases the likelihood of somaclonal variation, so the number of subcultures in micropropagation protocols should be kept to a minimum. Regular reinitiation of clones from new explants might reduce variability over time. Another way of reducing somaclonal variation is to avoid 2,4-D in the culture medium, as this hormone is known to introduce variation.Vitrification[hyperhydracity] may be a problem in some species. In case of forest trees, mature elite trees can be identified and rapidly cloned by this technique.
High production cost has limited the application of this technique to more valuable ornamental crops and some fruit trees.
5. Explain the principle and significance of embryo rescue techniques
Embryo Rescue is one of the earliest and successful forms of in-vitro culture techniques that is used to assist in the development of plant embryos that might not survive to become viable plants. Embryo rescue plays an important role in modern plant breeding, allowing the development of many interspecific and intergeneric food and ornamental plant crop hybrids. This technique nurtures the immature or weak embryo, thus allowing it the chance to survive. Plant embryos are multicellular structures that have the potential to develop into a new plant. The most widely used embryo rescue procedure is referred to as embryo culture, and involves excising plant embryos and placing them onto media culture. Embryo rescue is most often used to create interspecific and intergeneric crosses that would normally produce seeds which are aborted.
Interspecific incompatibility in plants can occur for many reasons, but most often embryo abortion occurs. In plant breeding, wide hybridization crosses can result in small shrunken seeds which indicate that fertilization has occurred, however the seed fails to develop. Many times, remote hybridizations will fail to undergo normal sexual reproduction, thus embryo rescue can assist in circumventing this problem.
Embryo rescue was first documented in the 18th century when Charles Bonnet excised Phaseolus and Fagopyrum embryos, planted them in soil and the cross resulted in dwarf plants. Soon after this, scientists began placing the embryos in various nutrient media. During the period of 1890 to
1904, systems for embryo rescue became systematic by applying nutrient solutions that contained salts and sugars and applying aseptic technique. The first successful in vitro embryo culture was performed by Hanning in 1904, he however described problems with precocious embryos that resulted in small, weak, and often inviable plantlets.
Applications
Breeding of incompatible interspecific and intergeneric species
Overcoming seed dormancy
Determination of seed viability
Recovery of maternal haploids that develop as a result of chromosome elimination following interspecific hybridization
Used in studies on the physiology of seed germination and development
Techniques
Depending on the organ cultured, it may be referred to as either embryo, ovule, or ovary culture.
Ovule culture or in ovolo embryo culture is a modified technique of embryo rescue whereby embryos are cultured while still inside their ovules to prevent damaging them during the excision process. Ovary or pod culture, on the other hand employs the use of an entire ovary into culture.
It becomes necessary to excise the entire small embryo to prevent early embryo abortion.
However, it is technically difficult to isolate the tiny intact embryos, so often ovaries with young embryos, or entire fertilized ovules will be used.
Factors to consider
Embryos are manually excised and placed immediately onto a culture media that provides the proper nutrients to support survival and growth (Miyajima 2006). While the disinfestation and explant excision processes differ for these three techniques, many of the factors that contribute to the successful recovery of viable plants are similar. The main factors that influence success are; the time of culture, the composition of the medium, and temperature and light. Timing mainly refers to the maturation stage of the embryo before excision. The optimal time especially for the rescue of embryos involving incompatible crosses would be just prior to embryo abortion.
Nevertheless, due to difficulties involved with the rearing of young embryos compared to those that have reached the autotrophic phase of development, embryos are normally allowed to develop in vivo as long as possible. While in general, two main types of basal media are the most commonly used for embryo rescue studies, i.e Murashige and Skoog medium (MS) and
Gamborg’s B-5 media (Bridgen, 1994), the composition of the media will vary in terms of the concentrations of media supplements required. This will generally depend on the stage of development of the embryo. For instance, young embryos would require a complex medium with high sucrose concentrations, while more mature embryos can usually develop on a simple medium with low levels of sucrose. The temperature and light requirement is generally species specific and thus its usually regulated to be the within the same temperature requirement as that of its parent with embryos of cool-season crops requiring lower temperatures than those of warm-season crops.
Part – C
1. Describe various stages involved in micro propagation techniques.
Micropropagation is the practice of rapidly multiplying stock plant material to produce a large number of progeny plants, using modern plant tissue culture methods.
Micropropagation is used to multiply novel plants, such as those that have been genetically modified or bred through conventional plant breeding methods. It is also used to provide a sufficient number of plantlets for planting from a stock plant which does not produce seeds, or does not respond well to vegetative reproduction.
Micropropagation begins with the selection of plant material to be propagated. Clean stock materials that are free of viruses and fungi are important in the production of the healthiest plants. Once the plant material is chosen for culture, the collection of explant(s) begins and is dependent on the type of tissue to be used; including stem tips, anthers, petals, pollen and others plant tissues. The explant material is then surface sterilized, usually in multiple courses of bleach and alcohol washes and finally rinsed in sterilized water. This small portion of plant tissue, sometimes only a single cell, is placed on a growth medium, typically containing sucrose as an energy source and one or more plant growth regulators (plant hormones). Usually the medium is thickened with agar to create a gel which supports the explant during growth. Some plants are easily grown on simple media but others require more complicated media for successful growth;
The plant tissue grows and differentiates into new tissues depending on the medium. For example, media containing cytokinins are used to create branched shoots from plant buds. and it happens in a vegetative form
Multiplication
Multiplication is the taking of tissue samples produced during the first stage and increasing their number. Following the successful introduction and growth of plant tissue, the establishment stage is followed by multiplication. Through repeated cycles of this process, a single explant sample may be increased from one to hundreds or thousands of plants. Depending on the type of tissue grown, multiplication can involve different methods and media. If the plant material grown is callus tissue, it can be placed in a blender and cut into smaller pieces and recultured on the same type of culture medium to grow more callus tissue. If the tissue is grown as small plants called plantlets, hormones are often added that cause the plantlets to produce many small offshoots that can be removed and recultured.
Pretransplant
This stage involves treating the plantlets/shoots produced to encourage root growth and
"hardening." It is performed in vitro, or in a sterile "test tube" environment.
"Hardening" refers to the preparation of the plants for a natural growth environment. Until this stage, the plantlets have been grown in "ideal" conditions, designed to encourage rapid growth.
Due to lack of necessity, the plants are likely to be highly susceptible to disease and often do not have fully functional dermal coverings and will be inefficient in their use of water and energy. In vitro conditions are high in humidity and plants grown under these condition do not form a working cuticle and stomata that keep the plant from drying out, when taken out of culture the plantlets need time to adjust to more natural environmental conditions. Hardening typically involves slowly weaning the plantlets from a high-humidity, low light, warm environment to what would be considered a normal growth environment for the species in question. This is done by moving the plants to a location high in humidity,
Transfer from culture
In the final stage of plant micropropagation, the plantlets are removed from the plant media and transferred to soil or (more commonly) potting compost for continued growth by conventional methods.
This stage is often combined with the "pretransplant" stage.
2. Write an essay on conventional breeding techniques
Plant breeding is defined as identifying and selecting desirable traits in plants and combining these into one individual plant. Since 1900, Mendel's laws of genetics provided the scientific basis for plant breeding. As all traits of a plant are controlled by genes located on chromosomes, conventional plant breeding can be considered as the manipulation of the combination of chromosomes. In general, there are three main procedures to manipulate plant chromosome combination. First, plants of a given population which show desired traits can be selected and used for further breeding and cultivation, a process called (pure line-) selection. Second, desired traits found in different plant lines can be combined together to obtain plants which exhibit both traits simultaneously, a method termed hybridization. Heterosis, a phenomenon of increased vigor, is obtained by hybridization of inbred lines. Third, polyploidy (increased number of chromosome sets) can contribute to crop improvement. Finally, new genetic variability can be introduced through spontaneous or artificially induced mutations.
Selection
Selection is the most ancient and basic procedure in plant breeding. It generally involves three distinct steps. First, a large number of selections are made from the genetically variable original population. Second, progeny rows are grown from the individual plant selections for observational purposes. After obvious elimination, the selections are grown over several years to permit observations of performance under different environmental conditions for making further eliminations. Finally, the selected and inbred lines are compared to existing commercial varieties in their yielding performance and other aspects of agronomic importance.
Hybridization
The most frequently employed plant breeding technique is hybridization. The aim of hybridization is to bring together desired traits found in different plant lines into one plant line via cross- pollination. The first step is to generate homozygous inbred lines. This is normally done by using self-pollinating plants where pollen from male flowers pollinates female flowers from the same plants. Once a pure line is generated, it is outcrossed, i. e. combined with another inbred line. Then the resulting progeny is selected for combination of the desired traits. If a trait from a wild relative of a crop species, e.g. resistance against a disease, is to be brought into the genome of the crop, a large quantity of undesired traits (like low yield, bad taste, low nutritional value) are transferred to the crop as well. These unfavorable traits must be removed by timeconsuming back-crossing, i. e. repeated crossing with the crop parent. There are two types of hybrid plants: interspecific and intergeneric hybrids. Beyond this biological boundary, hybridization cannot be accomplished due to sexual incompatibility, which limits the possibilities of introducing desired traits into crop Plants.
Heterosis is an effect which is achieved by crossing highly inbred lines of crop plants. Inbreeding of most crops leads to a strong reduction of vigor and size in the first generations. After six or seven generations, no further reduction in vigor or size is found. When such highly inbred plants are crossed with other inbred varieties, very vigorous, large sized, large-fruited plants may result.
The term "heterosis" is used to describe the phenomenon of hybrid vigor. The most notable and successful hybrid plant ever produced is the hybrid maize. By 1919, the first commercial hybrid maize was available in the United States. Two decades later, nearly all maize was hybrid, as it is today, although the farmers must buy new hybrid seed every year, because the heterosis effect is lost in the first generation after hybridization of the inbred parental lines.
Polyploidy
Most plants are diploid. Plants with three or more complete sets of chromosomes are common and are referred to as polyploids. The increase of chromosomes sets per cell can be artificially induced by applying the chemical colchicine, which leads to a doubling of the chromosome number. Generally, the main effect of polyploidy is increase in size and genetic variability. On the other hand, polyploid plants often have a lower fertility and grow more slowly.
Induced mutation
Instead of relying only on the introduction of genetic variability from the wild species gene pool or from other cultivars, an alternative is the introduction of mutations induced by chemicals or radiation. The mutants obtained are tested and further selected for desired traits. The site of the mutation cannot be controlled when chemicals or radiation are used as agents of mutagenesis.
Because the great majority of mutants carry undesirable traits, this method has not been widely used in breeding programs.
Success in using conventional plant breeding principles and agricultural techniques reached its peak when high-yielding wheat and rice lines were cultivated in the 1960s. The doubling and tripling of productivity of these important crops in Asia signaled a agricultural revolution in the developing countries. This breakthrough in food production was termed the "Green Revolution" to describe the social, economic, and nutritional impact of the new high-yielding wheat and rice strains. Norman Borlaug was awarded the Noble Price in 1970 for his contribution in breeding new high-yielding strains of cereals. However, these strains were highly dependent on fertilizers, irrigation and agrochemicals and required energy-intensive investments. This led to degradation and loss of soils and other severe environmental problems all over the world. For these reasons, the "Green Revolution" has been both praised and damned.
3. Write an essay on Biotechnological Methods of plant breeding
Biotechnology is the discipline which deals with the use of living organisms or their products. In this wide sense, also traditional agriculture may be seen as a form of biotechnology. The
European Federation of Biotechnology defines biotechnology as "the integrated use of biochemistry, microbiology and engineering sciences in order to achieve technological
(industrial) application of the capability of microorganisms, cultured tissue cells and parts thereof". In recent years, biotechnology has developed rapidly as a practical means for accelerating success in plant breeding and improving economically important crops. The most important methods used to achieve these goals are described below. The techniques of genetic
engineering, which are a part of biotechnology, will be discussed in more detail in the next chapter.
In Vitro Cultivation of Plant Cells and Regeneration of Plants from Cultured Cells Certain isolated somatic plant cells can be cultured in vitro (in the test tube) and are capable of proliferation and organization into tissues and eventually into complete plants. The process of regenerating whole plants out of plant cells is called in vitro regeneration. The three factors affecting plant regeneration are genotype, explant source, and culture conditions, including culture medium and environment. Different mixtures of plant hormones and other compounds in varying concentrations are used to achieve regeneration of plants from cultured cells and tissues.
As the plant hormonal mechanisms are not yet understood completely, the development of in vitro cultivation and regeneration systems is still largely based on empirically testing variations of the three above mentioned factors.
In Vitro Selection and Somaclonal Variation : Plants regenerated from cell cultures may exhibit phenotypes differing from their parent plants, sometimes at quite high frequencies. If these are heritable and affecting desirable agronomic traits, such "somaclonal variation" can be incorporated into breeding programs. However, finding of specific valuable traits by this method is largely left to chance and hence inefficient. Rather than relying on this undirected process, selection in vitro targets specific traits by subjecting large populations of cultured cells to the action of a selective agent in the Petri dish. For purposes of disease resistance, this selection can be provided by pathogens, or isolated pathotoxins that are known to have a role in pathogenesis.
The selection will only allow those cells to survive and proliferate that are resistant to the challenge. Selection of cells also plays an important role in genetic engineering, where special marker genes are used to select for transgenic cells.
Somatic Hybrid Plants : Somatic hybrid plants are plants derived from the fusion of somatic cells. Cell fusion was developed after the successful culture of a large number of plant cells stripped of their cell walls. The resulting cells without walls are referred to as protoplasts. Since also protoplasts from phylogenetically unrelated species can be fused, attempts have been made to overcome sexual incompatibility using protoplast fusion. In most cases, these attempts failed because growth and division of the fused cells did not take place when only distantly related cells were fused. Successful fusions between sexually incompatible petunia species and between potatoes and tomatoes did not lead to economically interesting products, but important contributions to the understanding of cell wall regeneration and other mechanisms were achieved
Genetic engineering is a term used for the directed manipulation of genes, i. e. the transfer of genes between organisms or changes in the sequence of a gene. Closely related to this field are methods which use genes or specific sequences for the identification of traits and other analytical purposes. In plant breeding, the most important and already widely used method of this kind is
Restriction Fragment Length Polymorphism (RFLP). The basics of this technique are described below. The principles of plant genetic engineering will be described in the next chapter. For those who are not familiar with the principles of genetics, the glossary at the end of this report explains the most important terms.
List down various applications of in vitro propagation techniques 4.
Micropropagation /Clonal Propagation
Clonal propagation refers to the process of asexual reproduction by multiplication of genetically identical copies of individual plants. The vegetative propagation of plants is labour-intensive, low in productivity and seasonal. The tissue culture methods of plant propagation, known as
'micropropagation' utilizes the culture of apical shoots, axillary buds and meristems on suitable nutrient medium.The regeneration of plantlets in cultured tissue was described by Murashige in
1974. Fossard (1987) gave a detailed account of stages of micropropagation.
The micropropagation is rapid and has been adopted for commercialization of important plants such as banana, apple, pears, strawberry, cardamom, many ornamentals (e.g. Orchids) and other plants.The micropropagation techniques are preferred over the conventional asexual propagation methods because of the following reasons: (a) In the micropropagation method, only a small amount of tissue is required to regenerate millions of clonal plants in a year., (b) micropropagation is also used as a method to develop resistance in many species., (c) in vitro stock can be quickly proliferated as it is season independent,. (d) long term storage of valuable germplasm possible.
The steps in micropropagation method are: a) Initiation of culture - from an explant like shoot tip on a suitable nutrient medium, b) multiple shoots formation from the cultured explant, c) rooting
of in vitro developed shoots and, d) transplantation - transplantation to the field following acclimatization.
The factors that affect micropropagation are: (a) genotype and the physiological status of the plant e.g. plants with vigorous germination are more suitable for micropropagation., (b) the culture medium and the culture environment like light, temperature etc. For example an illumination of 16 hours a day and 8 hours night is satisfactory for shoot proliferation and a temperature of 250C is optimal for the growth.
The benefits of micropropagation this method are: a) rapid multiplication of superior clones can be carried out through out the year, irrespective of seasonal variations. b) multiplication of disease free plants e.g. virus free plants of sweet potato (Ipomea batatus), cassava (Manihot esculenta) c) multiplication of sexually derived sterile hybrids d) It is a cost effective process as it requires minimum growing space.
Somaclonal variation
The genetic variations found in the in vitro cultured cells are collectively referred to as somaclonal variation and the plants derived from such cells are called as ‘somaclones’. It has been observed that the long-term callus and cell suspension culture and plants regenerated from such cultures are often associated with chromosomal variations. It is this property of cultured cells that finds potential application in the crop improvement and in the production of mutants and variants (e.g. disease resistance in potato).
Larkin and Scowcroft (1981) working at the division of Plant Industry, C.S.I.R.O., Australia gave the term 'somaclones' for plant variants obtained from tissue cultures of somatic tissues.
Similarly, if the tissue from which the variants have been obtained is having gametophytic origin such as pollen or egg cell, it is known as 'gametoclonal' variation.They explained that it may be due to: (a) reflection of heterogeneity between the cells and explant tissue, (b) a simple representation of spontaneous mutation rate, and (c) activation by culture environment of transposition of genetic materials.
Shepard et al. (1980) also contributed by screening about 100 somaclones produced from leaf protoplasts of Russet Burbank. They found that there was a significant amount of stable variation
in compactness of growth habit, maturity, date, tuber uniformity, tuber skin colour and photoperiodic requirements.
Somaclonal Variations has been used in plant breeding programmes where the genetic variations with desired or improved characters are introduced into the plants and new varieties are created that can exhibit disease resistance, improved quality and yield in plants like cereals, legumes, oil seeds tuber crops etc. Somaclonal variation is applicable for seed
Applications of Somaclonal Variations a) Methodology of introducing somaclonal variations is simpler and easier as compared to recombinant DNA technology. b) Development and production of plants with disease resistance e.g. rice, wheat, apple, tomato etc. c) Develop biochemical mutants with abiotic stress resistance e.g. aluminium tolerance in carrot, salt tolerance in tobacco and maize. d) Development of somaclonal variants with herbicide resistance e.g. tobacco resistant to sulfonylurea e) Development of seeds with improved quality e.g. a new variety of Lathyrus sativa seeds
(Lathyrus Bio L 212) with low content of neurotoxin. f) Bio-13 – A somaclonal variant of Citronella java (with 37% more oil and 39% more citronellon), a medicinal plant has been released as Bio-13 for commercial cultivation by Central
Institute for Medicinal and Aromatic Plants (CIMAP), Lucknow, India. g) Supertomatoes- Heinz Co. and DNA plant Technology Laboratories (USA) developed
Supertomatoes with high solid component by screening somaclones which helped in reducing the shipping and processing costs.
Production of virus free plants
The viral diseases in plants transfer easily and lower the quality and yield of the plants. It is very difficult to treat and cure the virus infected plants therefore te plant breeders are always interested in developing and growing virus free plants.
In some crops like ornamental plants, it has become possible to produce virus free plants through tissue culture at the commercial level. This is done by regenerating plants from cultured tissues derived from a) virus free plants, b) meristems which are generally free of infection - In the elimination of the virus, the size of the meristem used in cultures play a very critical role because most of the viruses exist by establishing a gradient in plant tissues. The regeneration of virus-free
plants through cultures is inversely proportional to the size of the meristem used., c) meristems treated with heat shock (34-360C) to inactivate the virus, d) callus, which is usually virus free like meristems.e) chemical treatment of the media- attempts have been made to eradicate the viruses from infected plants by treating the culture medium with chemicals e.g. addition of cytokinins suppressed the multiplication of certain viruses.
Among the culture techniques, meristem-tip culture is the most reliable method for virus and other pathogen elimination.
Viruses have been eliminated from a number of economically important plant species which has resulted in a significant increase in the yield and production e.g. potato virus X from potato, mosaic virus from cassava etc. These virus free plants are not disease resistant so there is a need to maintain stock plants to multiply virus free plants whenever required.
Production of synthetic seeds
In synthetic seeds, the somatic embryos are encapsulated in a suitable matrix (e.g. sodium alginate), along with substances like mycorrhizae, insecticides, fungicides and herbicides. These artificial seeds can be utilized for the rapid and mass propagation of desired plant species as well as hybrid varieties.
The major benefits of synthetic seeds are: a) They can be stored up to a year with out loss of viability b) Easy to handle and useful as units of delivery c) Can be directly sown in the soil like natural seeds and do not need acclimatization in green house.
Mutant selection
An important use of cell cultures is in mutant selection in relation to crop improvement. The frequency of mutations can be increased several fold through mutagenic treatments and millions of cells can be screened. A large number of reports are available where mutants have been selected at cellular level. The cells are often selected directly by adding the toxic substance against which resistance is sought in the mutant cells. Using this method, cell lines resistant to amino acid analogues, antibiotics, herbicides, fungal toxins etc have actually been isolated.
Production of secondary metabolites
The most important chemicals produced using cell culture are secondary metabolites, which are defined as’ those cell constituents which are not essential for survival’. These secondary metabolites include alkaloids, glycosides (steroids and phenolics), terpenoids, latex, tannins etc.
It has been observed that as the cells undergo morphological differentiation and maturation during plant growth, some of the cells specialize to produce secondary metabolites. The in vitro production of secondary metabolites is much higher from differentiated tissues when compared to non-differentiated tissues.
The cell cultures contribute in several ways to the production of natural products. These are: (a) a new route of synthesis to establish products e.g. codeine, quinine, pyrethroids, (b) a route of synthesis to a novel product from plants difficult to grow or establish e.g. thebain from Papaver bracteatum, (c) a source of novel chemicals in their own right e.g. rutacultin from culture of
Ruta, (d) as biotransformation systems either on their own or as part of a larger chemical process e.g. digoxin synthesis.
The advantages of in vitro production of secondary metabolites a) The cell cultures and cell growth are easily controlled in order to facilitate improved product formation. b) The recovery of the product is easy. c) As the cell culture systems are independent of environmental factors, seasonal variations, pest and microbial diseases, geographical location constraints, it is easy to increase the production of the required metabolite. d) Mutant cell lines can be developed for the production of novel and commercially useful compounds. e) Compounds are produced under controlled conditions as per the market demands. f) The production time is less and cost effective due to minimal labour involved.
Production of Somatic hybrids and cybrids
The Somatic cell hybridization/ parasexual hybridization or Protoplast fusion offers an alternative method for obtaining distant hybrids with desirable traits significantly between species or genera, which can not be made to cross by conventional method of sexual hybridization.
Somatic hybridization
Somatic hybridization broadly involves in vitro fusion of isolated protoplasts to form a hybrid cell and its subsequent development to form a hybrid plant. The process involves: a) fusion of protoplasts, (b) Selection of hybrid cells, (c) identification of hybrid plants.
During the last two decades, a variety of treatments have been used to bring about the fusion of plant protoplasts. Protoplast fusion can be achieved by spontaneous, mechanical, or induced fusion methods.. These treatments include the use of fusogens like NaNO3, high pH with high
Ca2++ ion concentration, use of polyethylene glycol (PEG), and electrofusion. These inducing agents used in protoplast fusion are called ‘fusogen’.
PEG treatment is the most widely used method for protoplast fusion as it has certain advantages over others. These are : (a) it results in a reproducible high-frequency of heterokaryon formation., (b) The PEG fusion is non specific and therefore can be used for a wide range of plants., (c) It has low toxicity to the cell and (d) The formation of binucleate heterokaryons is low.
Mechanism of fusion : The fusion of protoplasts takes place in three phases- agglutination, plasma membrane fusion and formation of heterokaryons. When the two protoplasts come in close contact with each other, they adhere to each other. This agglutination can be induced by
PEG, high pH and high Ca2+. The protoplast membranes get fused at localized sites at the point of adhesion. This leads to the formation of cytoplasmic bridges between protoplasts. High pH and high Ca2+ ions neutralize the surface charges on the protoplasts which allows closer contact and membrane fusion between agglutinated protoplasts. The fused protoplasts become round as a result of cytoplasmic bridges which leads to the formation of spherical homokaryon or heterokaryon.
Selection of hybrid cells : The methods used for the selection of hybrid cells are biochemical, visual and cytometric methods using fluorescent dyes. The biochemical methods for selection of hybrid cells are based on the use of biochemical compounds in the medium. The drug sensitivity method is useful for the selection hybrids of two plants species, if one of them is sensitive to a drug. Another method, auxotrophic mutant selection method involves the auxotrophs which are mutants that cannot grow on a minimal medium. Therefore specific compounds are added in the medium. The selection of auxotropic mutants is possible only if the hybrid cells can grow on a minimal medium. The visual method involves the identification of heterokaryons under the light microscope. In some of the somatic hybridizations, the chloroplast deficient protoplast of one plant species is fused with the green protoplast of another plant species. The heterokaryons obtained are bigger and green in colour while the parental protoplasts are either small or colourless. The cytometric method uses flow cytometry and flourescent-activated cell sorting techniques for the analysis of plant protoplasts.
Applications of Somatic hybridization a) Creation of hybrids with disease resistance - Many disease resistance genes (e.g. tobacco mosaic virus, potato virus X, club rot disease) could be successfully transferred from one species
to another. E.g resistance has been introduced in tomato against diseases such as TMV, spotted wilt virus and insect pests. b) Environmental tolerance - using somatic hybridization the genes conferring tolerance for cold, frost and salt were introduced in e.g. in tomato. c) Cytoplasmic male sterility - using cybridization method, it was possible to transfer cytoplasmic male sterility. d) Quality characters - somatic hybrids with selective characteristics have been developed e.g. the production of high nicotine content.
Chromosome number in somatic hybrids : The chromosome number in the somatic hybrids is generally more than the total number of both of the parental protoplasts. If the chromosome number in the hybrid is the sum of the chromosomes of the two parental protoplasts, the hybrid is said to be symmetric hybrid. Asymmetric hybrids have abnormal or wide variations in the chromosome number than the exact total of two species.
In 1972, Carlson and his associates produced the first inter-specific somatic hybrid between
Nicotiana glauca and N. langsdorffii. In 1978, Melchers and his co-workers developed the first inter-genetic somatic hybrids between Solanum tuberosum (potato) and Lycopersicon esculentum (tomato). The hybrids are known as ‘Pomatoes or Topatoes’.
Limitations of Somatic Hybridization : a) Somatic hybridization does not always produce plants that give fertile and visible seeds. b) There is genetic instability associated with protoplast culture. c) There are limitations in the selection methods of hybrids, as many of them are not efficient. d) Somatic hybridization between two diploids results in the formation of an amphidiploid which is not favourable therefore haploid protoplasts are recommended in somatic hybridization. e) It is not certain that a specific character will get expressed in somatic hybridization. f) Regenerated plants obtained from somatic hybridization are often variable due to somaclonal variations, chromosomal elimination, organelle segregation etc. g) Protoplast fusion between different species/genus is easy, but the production of viable somatic hybrids is not always possible.
Cybrids
The cytoplasmic hybrids where the nucleus is derived from only one parent and the cytoplasm is derived from both the parents are referred to as cybrids. The process of formation of cybrids is called cybridization. During the process of cybridization and heterokaryon formation, the nuclei
are stimulated to segregate so that one protoplast contributes to the cytoplasm while the other contributes nucleus alone. The irradiation with gamma rays and X-rays and use of metabolic inhibitors makes the protoplasts inactive and non-dividing. Some of the genetic traits in certain plants are cytoplasmically controlled. This includes certain types of male sterility, resistance to certain antibiotics and herbicides. Therefore cybrids are important for the transfer of cytoplasmic male sterility (CMS), antibiotic and herbicide resistance in agriculturally useful plants. Cybrids of Brassica raphanus that contain nucleus of B. napus, chloroplasts of atrazinc resistant B. capestris and male sterility from Raphanus sativas have been developed.
In vitro plant germplasm conservation
Germplasm refers to the sum total of all the genes present in a crop and its related species.
The conservation of germplasm involves the preservation of the genetic diversity of a particular plant or genetic stock for it’s use at any time in future. It is important to conserve the endangered plants or else some of the valuable genetic traits present in the existing and primitive plants will be lost. A global organization- International Board of Plant Genetic Resources (IBPGR) has been established for germplasm conservation and provides necessary support for collection, conservation and utilization of plant geneic resources through out the world. The germplasm is preserved by the following two ways:
(a) In-situ conservation- The germplasm is conserved in natural environment by establishing biosphere reserves such as national parks, sanctuaries. This is used in the preservation of land plants in a near natural habitat along with several wild types.
(b) Ex-situ conservation- This method is used for the preservation of germplasm obtained from cultivated and wild plant materials. The genetic material in the form of seeds or in vitro cultures are preserved and stored as gene banks for long term use.
In vivo gene banks have been made to preserve the genetic resources by conventional methods e.g. seeds, vegetative propagules, etc. In vitro gene banks have been made to preserve the genetic resources by non - conventional methods such as cell and tissue culture methods. This will ensure the availability of valuable germplasm to breeder to develop new and improved varieties.
The methods involved in the in vitro conservation of germplasm are:
(a) Cryopreservation- In cryopreservation (Greek-krayos-frost), the cells are preserved in the frozen state. The germplasm is stored at a very low temperature using solid carbon dioxide (at -
790C), using low temperature deep freezers (at -800C), using vapour nitrogen (at- 1500C) and liquid nitrogen (at-1960C). The cells stay in completely inactive state and thus can be conserved for long periods. Any tissue from a plant can be used for cryopreservation e.g. meristems, embryos, endosperms, ovules, seeds, cultured plant cells, protoplasts, calluses. Certain compounds like- DMSO (dimethyl sulfoxide), glycerol, ethylene, propylene, sucrose, mannose, glucose, praline, acetamide etc are added during the cryopreservation. These are called
cryoprotectants and prevent the damage caused to cells (by freezing or thawing) by reducing the freezing point and super cooling point of water.
(b) Cold Storage- Cold storage is a slow growth germplasm conservation method and conserves the germplasm at a low and non-freezing temperature (1-90C). The growth of the plant material is slowed down in cold storage in contrast to complete stoppage in cryopreservation and thus prevents cryogenic injuries. Long term cold storage is simple, cost effective and yields germplasm with good survival rate. Virus free strawberry plants could be preserved at 100C for about 6 years. Several grape plants have been stored for over 15 years by using a cold storage at temperature around 90C and transferring them in the fresh medium every year.
(c) Low pressure and low oxygen storage- In low- pressure storage, the atmospheric pressure surrounding the plant material is reduced and in the low oxygen storage, the oxygen concentration is reduced. The lowered partial pressure reduces the in vitro growth of plants. In the low-oxygen storage, the oxygen concentration is reduced and the partial pressure of oxygen below 50 mmHg reduces plant tissue growth. Due to the reduced availability of O2, and reduced production of CO2, the photosynthetic activity is reduced which inhibits the plant tissue growth and dimension. This method has also helped in increasing the shelf life of many fruits, vegetables and flowers.
The germplasm conservation through the conventional methods has several limitations such as short-lived seeds, seed dormancy, seed-borne diseases, and high inputs of cost and labour. The techniques of cryo-preservation (freezing cells and tissues at -1960c) and using cold storages help us to overcome these problems.
5. Explain the principles of isolation and culture of protoplasts
Isolation of protoplasts
Protoplasts (cell minus cell wall) is the biologically active and most significant material of cells.
When cell wall is mechanically or enzymatically removed the isolated protoplast is known as
"naked plant cell" on which most of recent researches are based.
Plant cell wall acts as physical barrier and protects cytoplasm from microbial invasion and environmental stress. It consists of a complex mixture of cellulose, hemicellulose, pectin, lignin, lipids, protein, etc. For dissolution of different components of the cell wall it is essential to have the respective enzymes.
Until suitable methods were developed, protoplasts were isolated by cutting the plasmolysed plant tissues and releasing protoplast through deplasmolysis of cells. Cooking (1960) for the first time isolated the protoplasts of plant tissues by using cell wall degrading enzymes viz., cellulase, hemicellulase, pectinase, and protease extracted from a saprophytic fungus Trichoderma viride.
Later on protoplasts were cultured in vitro.
Microorganisms are well equipped with a system to produce substrate specific extracellular enzymes, the extent of which depends on the genetic variability of the specific species and strains. A detailed account of enzyme preparation and their uses in isolation of protoplast has been given by Cooking (1972 ); Bajaj (1977b) and Patnaik et al (1981). However, the basic techniques of isolation and culture of protoplast are given in Figs. 8.5. and 8.6 with a brief description.
Surface Sterilization of Leaf Samples : Mature leaves are collected from healthy plants which are washed in tap water to remove adhering soil particles and sterilized with sodium hypochlorite solution.
Rinsing in Suitable Osmoticum : After 10 min, sample is properly washed with sterile distilled water or MS medium adjusted to a suitable pH and buffer to maintain osmotic pressure. Washing should be done for about 6 times to remove the traces of sodium hypochlorite.
Plasmolysis of Cells : The lower epidermis covered by thin wax cuticle is removed with a forcep.
Stripping should be done from midrib to margin of lamina. The stripped surface of leaf is kept in mannitol solution (13% W/V) for 3 hours to allow plasmolysis of cells.
Peeling of Lower Epidermis : Thereafter, about 1 gm leaves are peeled off and transferred into enzyme mixture already sterilized through a Seitz filter (0.45 mm). This facilitates the penetration of enzyme into tissue within 12-18 hours at 25°C.
Isolation and Purification of Portoplasts : Leaf debris are removed with forcep, and enzyme solution containing protoplasts are filtered with a nylon mesh (45mm). Filtrate is centrifuged at
75 X g for 5 min and supernatant is decanted. Again a fresh MS medium plus 13% mannitol is added to centrifuge. Repeated washing with nutrient medium, centrifugation and decantation are done for about three time. Finally specific concentration of protoplast suspension is prepared.
Protoplast culture and regeneration
From the protoplast solution of known density (about 105 protoplast/ml) about 1 ml suspension is poured on sterile and cooled down nutrient medium in Petri dishes. The plates are incubated at
25°C in a dim white light.
The protoplasts regenerate a cell wall, undergo cell division and form callus. The callus can also be subcultured. Embryogenesis begins from callus when it is placed on nutrient medium lacking mannitol and auxin. The embryo develops into seedlings and finally mature plants
6. Explain somatic embryogenesis with suitable diagrams
Somatic embryos are mainly produced in vitro and for laboratory purposes, using either solid or liquid nutrient media which contain plant growth regulators (PGR’s). The main PGRs used are
auxins but can contain cytokinin in a smaller amount. Somatic embryogenesis is a process where a plant or embryo is derived from a single somatic cell or group of somatic cells. This is in contrast to zygotic embryogenesis, where ,in diploid species, two haploid cells combine to form one diploid cell. Somatic embryos are produced when somatic cells are restructured through a series of morphological and biochemical changes in the embryogenic pathway. Development of somatic embryos is not that different from zygotic embryos. Three examples of somatic embryogenesis from nature are ovules in Paeonia and on the leaves of Asplenium and
Kalanchoe. Shoots and roots are monopolar while somatic embryos are bipolar, allowing them to form a whole plant without culturing on multiple media types. Somatic embryogenesis has served as a model to understand the physiological and biochemical events that occur plant developmental processes as well as a component to biotechnological advancement. The first documentation of somatic embryogenesis was by Steward et al in 1958 and Reinert in 1959 with carrot cell suspension cultures.
7. Explain haploid culture techniques and describe their advantages
Anther and Pollen Culture (Production of Haploid Plants) :
Anther, a male reproductive organ, is diploid in chromosome numbers. As a result of microsporogenesis, tetrads of microspores are formed from a single spore mother cell. They are known as pollen grains after release from tetrads (Bhojwani and Bhatnagar, 1974). The aim of anther and pollen culture is to get haploid plants by induction of embryogenesis. Haploid plants have single complete set of chromosomes that in turn may be useful for the improvement of many crop plants (Sunderland, 1979). Moreover, chromosome set of these haploids can be doubled by mutagenic chemicals (e.g. colchicine) or regeneration technique to obtain fertile homozygous diploi4Su(Vasil and Nitsch, 1975).
Tulecke (1951) cultured pollen grains of Ginkgo biloba (gymnosperm) and succeeded to induce the development of haploid callus. Guha and Maheswari (1964) made a remarkable discovery by culturing pollen grains of an angiospermic plant, Datura innoxia on the nutrient agar medium and also developed torpedo- shaped embryoids that metamorphosed into plantlets through the process.
Sunderland (1979) has described that the anthers to be cultured should be one of the three categories i.e. premitotic, mitotic and postmitotic. In premitotic anthers, where the microspores have completed meiosis but not started first pollen division, the best response is achieved e.g.
Hordeum vulgare. In mitotic anthers where first pollen division has started the optimum responses are achieved e.g. N. tabacum and D. innoxia. In post mitotic anthers, the early bicellular stage of pollen development is the best time to culture e.g. Atropa belladona.
Haploid plants are very useful in (i) direct screening of recessive mutation because in diploid or polyploid screening of recessive mutation is not possible, and (ii) development of homozygous diploid plants following chromosome doubling of haploid plant cells.
In China, the most widely grown wheat is a doubled haploid produced through homozygous diploid lines. Anther culture of rice is also successfully grown. Haploid plants have been produced in tobacco, wheat and rice through pollen culture. These are used for the development of disease resistant and superior diploid lines.
At present, more than 247 plant species and hybrids belonging to 38 genera and 34 families of dicots and monocots have been regenerated using anther culture technique. They include economically important crops and trees such as rice, wheat maize, coconut, rubber trees, etc,
(Maheswari et al.1983).
8. Describe plant growth regulators with their characteristic feature
Plant hormones (also known as phytohormones) are chemicals that regulate plant growth, which, in the UK, are termed 'plant growth substances'. Plant hormones are signal molecules produced within the plant, and occur in extremely low concentrations. Hormones regulate cellular processes in targeted cells locally and when moved to other locations, in other locations of the plant. Hormones also determine the formation of flowers, stems, leaves, the shedding of leaves, and the development and ripening of fruit. Plants, unlike animals, lack glands that produce and secrete hormones, instead each cell is capable of producing hormones. Plant hormones shape the plant, affecting seed growth, time of flowering, the sex of flowers, senescence of leaves and fruits. They affect which tissues grow upward and which grow downward, leaf formation and stem growth, fruit development and ripening, plant longevity, and even plant death. Hormones are vital to plant growth and lacking them, plants would be mostly a mass of undifferentiated cells.
Characteristics
The word hormone is derived from Greek, meaning 'set in motion.' Plant hormones affect gene expression and transcription levels, cellular division, and growth. They are naturally produced within plants, though very similar chemicals are produced by fungi and bacteria that can also affect plant growth. A large number of related chemical compounds are synthesized by humans, they are used to regulate the growth of cultivated plants, weeds, and in vitro-grown plants and plant cells; these manmade compounds are called Plant Growth Regulators or PGRs for short.
Early in the study of plant hormones, "phytohormone" was the commonly used term, but its use is less widely applied now.
Plant hormones are not nutrients, but chemicals that in small amounts promote and influence the growth, development, and differentiation of cells and tissues. The biosynthesis of plant hormones within plant tissues is often diffuse and not always localized. Plants lack glands to produce and
store hormones, because, unlike animals, which have two circulatory systems (lymphatic and cardiovascular) powered by a heart that moves fluids around the body, plants use more passive means to move chemicals around the plant. Plants utilize simple chemicals as hormones, which move more easily through the plant's tissues. They are often produced and used on a local basis within the plant body, plant cells even produce hormones that affect different regions of the cell producing the hormone.
Hormones are transported within the plant by utilizing four types of movements. For localized movement, cytoplasmic streaming within cells and slow diffusion of ions and molecules between cells are utilized. Vascular tissues are used to move hormones from one part of the plant to another; these include sieve tubes that move sugars from the leaves to the roots and flowers, and xylem that moves water and mineral solutes from the roots to the foliage.
Not all plant cells respond to hormones, but those cells that do are programmed to respond at specific points in their growth cycle. The greatest effects occur at specific stages during the cell's life, with diminished effects occurring before or after this period. Plants need hormones at very specific times during plant growth and at specific locations. They also need to disengage the effects that hormones have when they are no longer needed. The production of hormones occurs very often at sites of active growth within the meristems, before cells have fully differentiated.
After production they are sometimes moved to other parts of the plant where they cause an immediate effect or they can be stored in cells to be released later. Plants use different pathways to regulate internal hormone quantities and moderate their effects; they can regulate the amount of chemicals used to biosynthesize hormones. They can store them in cells, inactivate them, or cannibalise already-formed hormones by conjugating them with carbohydrates, amino acids or peptides. Plants can also break down hormones chemically, effectively destroying them. Plant hormones frequently regulate the concentrations of other plant hormones. Plants also move hormones around the plant diluting their concentrations.
The concentration of hormones required for plant responses are very low (10−6 to 10−5 mol/L).
Because of these low concentrations, it has been very difficult to study plant hormones, and only since the late 1970s have scientists been able to start piecing together their effects and relationships to plant physiology. Much of the early work on plant hormones involved studying plants that were genetically deficient in one or involved the use of tissue-cultured plants grown in vitro that were subjected to differing ratios of hormones, and the resultant growth compared.
The earliest scientific observation and study dates to the 1880s; the determination and observation of plant hormones and their identification was spread-out over the next 70 years.
Classes of plant hormonesIn general, it is accepted that there are five major classes of plant hormones, some of which are made up of many different chemicals that can vary in structure from one plant to the next. The chemicals are each grouped together into one of these classes based on their structural similarities and on their effects on plant physiology. Other plant hormones and growth regulators are not easily grouped into these classes; they exist naturally or are synthesized by humans or other organisms, including chemicals that inhibit plant growth or
interrupt the physiological processes within plants. Each class has positive as well as inhibitory functions, and most often work in tandem with each other, with varying ratios of one or more interplaying to affect growth regulation.
The five major classes are:
Abscisic acid
Abscisic acid also called ABA, was discovered and researched under two different names before its chemical properties were fully known, it was called dormin and abscicin II. Once it was determined that the two latter compounds were the same; it was named abscisic acid. The name
"abscisic acid" was given because it was found in high concentrations in newly abscissed or freshly fallen leaves.
This class of PGR is composed of one chemical compound normally produced in the leaves of plants, originating from chloroplasts, especially when plants are under stress. In general, it acts as an inhibitory chemical compound that affects bud growth, seed and bud dormancy. It mediates changes within the apical meristem causing bud dormancy and the alteration of the last set of leaves into protective bud covers. Since it was found in freshly abscissed leaves, it was thought to play a role in the processes of natural leaf drop but further research has disproven this. In plant species from temperate parts of the world it plays a role in leaf and seed dormancy by inhibiting growth, but, as it is dissipated from seeds or buds, growth begins. In other plants, as ABA levels decrease, growth then commences as gibberellin levels increase. Without ABA, buds and seeds would start to grow during warm periods in winter and be killed when it froze again. Since ABA dissipates slowly from the tissues and its effects take time to be offset by other plant hormones, there is a delay in physiological pathways that provide some protection from premature growth.
It accumulates within seeds during fruit maturation, preventing seed germination within the fruit, or seed germination before winter. Abscisic acid's effects are degraded within plant tissues during cold temperatures or by its removal by water washing in out of the tissues, releasing the seeds and buds from dormancy.
In plants under water stress, ABA plays a role in closing the stomata. Soon after plants are waterstressed and the roots are deficient in water, a signal moves up to the leaves, causing the formation of ABA precursors there, which then move to the roots. The roots then release ABA, which is translocated to the foliage through the vascular system and modulates the potassium and sodium uptake within the guard cells, which then lose turgidity, closing the stomata. ABA exists in all parts of the plant and its concentration within any tissue seems to mediate its effects and function as a hormone; its degradation, or more properly catabolism, within the plant affects metabolic reactions and cellular growth and production of other hormones. Plants start life as a seed with high ABA levels, just before the seed germinates ABA levels decrease; during germination and early growth of the seedling, ABA levels decrease even more. As plants begin to produce shoots with fully functional leaves - ABA levels begin to increase, slowing down cellular growth in more "mature" areas of the plant. Stress from water or predation affects ABA
production and catabolism rates, mediating another cascade of effects that trigger specific responses from targeted cells. Scientists are still piecing together the complex interactions and effects of this and other phytohormones.
Auxins
The auxin indoleacetic acidAuxins are compounds that positively influence cell enlargement, bud formation and root initiation. They also promote the production of other hormones and in conjunction with cytokinins, they control the growth of stems, roots, and fruits, and convert stems into flowers.[11] Auxins were the first class of growth regulators discovered.[12] They affect cell elongation by altering cell wall plasticity. Auxins decrease in light and increase where it is dark. They stimulate cambium, a subtype of meristem cells, to divide and in stems cause secondary xylem to differentiate. Auxins act to inhibit the growth of buds lower down the stems
(apical dominance), and also to promote lateral and adventitious root development and growth.
Leaf abscission is initiated by the growing point of a plant ceasing to produce auxins. Auxins in seeds regulate specific protein synthesis,[13] as they develop within the flower after pollination, causing the flower to develop a fruit to contain the developing seeds. Auxins are toxic to plants in large concentrations; they are most toxic to dicots and less so to monocots. Because of this property, synthetic auxin herbicides including 2,4-D and 2,4,5-T have been developed and used for weed control. Auxins, especially 1-Naphthaleneacetic acid (NAA) and Indole-3-butyric acid
(IBA), are also commonly applied to stimulate root growth when taking cuttings of plants. The most common auxin found in plants is indoleacetic acid or IAA. The correlation of auxins and cytokinins in the plants is a constant (A/C = const.).
Cytokinins
The cytokinin zeatin , Zea, in which it was first discovered in immature kernels.Cytokinins or
CKs are a group of chemicals that influence cell division and shoot formation. They were called kinins in the past when the first cytokinins were isolated from yeast cells. They also help delay senescence or the aging of tissues, are responsible for mediating auxin transport throughout the plant, and affect internodal length and leaf growth. They have a highly synergistic effect in concert with auxins and the ratios of these two groups of plant hormones affect most major growth periods during a plant's lifetime. Cytokinins counter the apical dominance induced by auxins; they in conjunction with ethylene promote abscission of leaves, flower parts and fruits.
The correlation of auxins and cytokinins in the plants is a constant (A/C = const.).
Ethylene
EthyleneEthylene is a gas that forms through the Yang Cycle from the breakdown of methionine, which is in all cells. Ethylene has very limited solubility in water and does not accumulate within the cell but diffuses out of the cell and escapes out of the plant. Its effectiveness as a plant hormone is dependent on its rate of production versus its rate of escaping into the atmosphere.
Ethylene is produced at a faster rate in rapidly growing and dividing cells, especially in darkness.
New growth and newly germinated seedlings produce more ethylene than can escape the plant,
which leads to elevated amounts of ethylene, inhibiting leaf expansion (see Hyponastic response). As the new shoot is exposed to light, reactions by phytochrome in the plant's cells produce a signal for ethylene production to decrease, allowing leaf expansion. Ethylene affects cell growth and cell shape; when a growing shoot hits an obstacle while underground, ethylene production greatly increases, preventing cell elongation and causing the stem to swell. The resulting thicker stem can exert more pressure against the object impeding its path to the surface.
If the shoot does not reach the surface and the ethylene stimulus becomes prolonged, it affects the stems natural geotropic response, which is to grow upright, allowing it to grow around an object. Studies seem to indicate that ethylene affects stem diameter and height: When stems of trees are subjected to wind, causing lateral stress, greater ethylene production occurs, resulting in thicker, more sturdy tree trunks and branches. Ethylene affects fruit-ripening: Normally, when the seeds are mature, ethylene production increases and builds-up within the fruit, resulting in a climacteric event just before seed dispersal. The nuclear protein Ethylene Insensitive2 (EIN2) is regulated by ethylene production, and, in turn, regulates other hormones including ABA and stress hormones.
Gibberellins
Gibberellin A1 Gibberellins, or GAs, include a large range of chemicals that are produced naturally within plants and by fungi. They were first discovered when Japanese researchers, including Eiichi Kurosawa, noticed a chemical produced by a fungus called Gibberella fujikuroi that produced abnormal growth in rice plants. Gibberellins are important in seed germination, affecting enzyme production that mobilizes food production used for growth of new cells. This is done by modulating chromosomal transcription. In grain (rice, wheat, corn, etc.) seeds, a layer of cells called the aleurone layer wraps around the endosperm tissue. Absoption of water by the seed causes production of GA. The GA is transported to the aleurone layer, which responds by producing enzymes that break down stored food reserves within the endosperm, which are utilized by the growing seedling. GAs produce bolting of rosette-forming plants, increasing internodal length. They promote flowering, cellular division, and in seeds growth after germination. Gibberellins also reverse the inhibition of shoot growth and dormancy induced by
ABA.
Unit II
Cell culture methods for the secondary metabolite production, somaclonal variation and its significance, Cryopreservation, Gene banks for germplasm conservation. Plant transformation techniques – Mechanism of DNA transfer – Agrobacterium mediated gene transfer, general features of TI and RI plasmids and their use as vectors, role of virulence genes; design of expression vectors; use of 35S and other promoters, reporter genes.
Part – A
1. Selection marker
A selectable marker is a gene introduced into a cell, especially a bacterium or to cells in culture, that confers a trait suitable for artificial selection. They are a type of reporter gene used in laboratory microbiology, molecular biology, and genetic engineering to indicate the success of a transfection or other procedure meant to introduce foreign DNA into a cell. Selectable markers are often antibiotic resistance genes; bacteria that have been subjected to a procedure to introduce foreign DNA are grown on a medium containing an antibiotic, and those bacterial colonies that can grow have successfully taken up and expressed the introduced genetic material.
2. GUS
The GUS reporter system (GUS: beta-glucuronidase) is a reporter gene system, particularly useful in plant molecular biology. [1] Several kinds of GUS reporter gene assay are actually available, depending on the substrate used. The term GUS staining refers to the most common of these, a histochemical technique
3. Reporter gene
In molecular biology, a reporter gene (often simply reporter) is a gene that researchers attach to a regulatory sequence of another gene of interest in cell culture, animals or plants. Certain genes are chosen as reporters because the characteristics they confer on organisms expressing them are easily identified and measured, or because they are selectable markers. Reporter genes are often used as an indication of whether a certain gene has been taken up by or expressed in the cell or organism population.
4. Binary vector
Used to generate transgenic plants, binary vectors are cloning vectors which are able to replicate in both E. coli and Agrobacterium tumefaciens, which are bacteria that are often used in biotechnology.
Systems in which T-DNA and vir genes are located on separate replicons are called T-DNA binary systems. T-DNA is located on the binary vector (the non-T-DNA region of this vector containing origin(s) of replication that could function both in E. coli and in Agrobacterium tumefaciens, and antibiotic-resistance genes used to select for the presence of the binary vector
in bacteria, became known as vector backbone sequences). The replicon containing the vir genes became known as the vir helper. Strains harboring this replicon and a T-DNA are considered disarmed if they do not contain oncogenes that could be transferred to a plant.
There are several binary vector systems that differ mainly in the plasmid region that facilitates replication in Agrobacterium. Commonly used binary vectors include: pBIN19, pPVP, pGreen.
5. Acetosyringone
Acetosyringone is a volatile chemical compound related to acetophenone. It is a natural product that can be found in Posidonia oceanica. The compound is also produced by the male leaffooted bug (Leptoglossus phyllopus) and used in its communication system.
This substance is involved in plant-pathogen recognition. The VirA gene of Ti plasmid, part of the genetic equipment that Agrobacterium tumefaciens and Agrobacterium rhizogenes soil bacteria use to infect plants, codes for a receptor to acetosyringone leaking from plant wounds.
This compound allows higher transformation efficiency in plants
6. Crown gall tumour
Protein involved in crown gall tumor formation, a plant tumor caused by the bacterium
Agrobacterium tumefaciens
Genetic colonization 7.
Natural introduction of genetic material into the deoxyribonucleic acid of a host cell; for example, transmission of a tumor-inducing plasmid into a plant cell by the bacterium
Agrobacterium tumefaciens.
8. Liposome
Liposomes are artificially prepared vesicles made of lipid bilayer. Liposomes can be filled with drugs, and used to deliver drugs for cancer and other diseases.[2] Liposomes can be prepared by disrupting biological membranes, for example by sonication.
Liposomes can be composed of naturally-derived phospholipids with mixed lipid chains (like egg phosphatidylethanolamine) or other surfactants. Liposomes should not be confused with micelles and reverse micelles composed of monolayers
Part – B
1. Explain the role of vir genes in Ti plasmid.
The T-DNA transfer is mediated by products encoded by the 30-40 kb vir region of the Ti plasmid. This region is composed by at least six essential operons (vir A, vir B, vir C, vir D, vir
E, virG ) and two non-essential (virF, virH). The number of genes per operon differs, virA, virG and virF have only one gene; virE, virC, virH have two genes while virD and virB have four and eleven genes respectively. The only constitutive expressed operons are virA and virG, coding for a two-component (VirA-VirG) system activating the transcription of the other vir genes. The
VirA-VirG two component system has structural and functional similarities to other already described for other cellular mechanisms (Nixon, 1986, Iuchi, 1993).
VirA is a transmembrane dimeric sensor protein that detects signal molecules, mainly small phenolic compounds, released from wounded plants (Pan et al., 1993). The signals for VirA activation include acidic pH, phenolic compounds, such as acetosyringone (Winans et al., 1992), and certain class of monosaccharides which acts sinergistically with phenolic compounds
(Ankenbauer et al., 1990; Cangelosi et al., 1990; Shimoda et al., 1990; Doty et al., 1996). VirA protein can be structurally defined into three domains: the periplasmic or input domain and two transmembrane domains (TM1 and TM2). The TM1 and TM2 domains act as a transmitter
(signaling) and receiver (sensor) (Parkinson, 1993). The periplasmic domain is important for monosaccharide detection (Chang and Winans, 1992). Within the periplasmic domain, adjacent to the TM2 domain is an amphipatic helix, with strong hydrophilic and hydrophobic regions
(Heath et al., 1995). This structure is characteristic for other transmembrane sensor proteins and folds the protein to be simultaneously aligned with the inner membrane and anchored in the membrane (Seligman and Manoil, 1994). The TM2 is the kinase domain and plays a crucial role in the activation of VirA, phosphorylating itself on a conserved His-474 residue (Huang et al.,
1990; Jin et al. 1990ª, 1990b) in response to signaling molecules from wounded plant sites.
Monosaccharide detection by VirA is an important amplification system and responds to low levels of phenolic compounds. The induction of this system is only possible through the periplasmic sugar (glucose/galactose) binding protein ChvE (Ankenbauer and Nester, 1990;
Cangelosi et al., 1990), which interacts with VirA (Shimoda et al., 1990, 1993; Turk et al., 1993;
Chang and Winans, 1992). Recent studies for determination of VirA regions, important for its sensing activity suggested the position, which may be involved on TM1-TM2 interaction. This interaction causes the exposure of the amphipathic helix to small phenolic compounds and suggests a putative model for the VirA-ChvE interaction (Doty et al., 1996).
Activated VirA has the capacity to transfer its phosphate to a conserved aspartate residue of the cytoplasmic DNA binding protein VirG (Jin et al. 1990a, 1990b; Pan et al., 1993). VirG functions as a transcriptional factor regulating the expression of vir genes when it is phosphorylated by VirA (Jin et al., 1990a, 1990b). The C-terminal region is responsible for the
DNA binding activity, while the N-terminal is the phosphorylation domain and shows homology with the VirA receiver (sensor) domain.
The activation of vir system also depends on external factors like temperature and pH. At temperatures greater than 32°C, the vir genes are not expressed because of a conformational change in the folding of VirA induce the inactivation of its properties (Jin et al., 1993). The effect of temperature on VirA is suppressed by a mutant form of VirG (VirGc), which activates the constitutive expression of the vir genes. However, this mutant cannot confers the virulence capacity at that temperature to Agrobacterium, probably because the folding of other proteins that actively participate in the T-DNA transfer process are also affected at high temperature
(Fullner and Nester, 1996).
2. Explain: Direct gene transfer.
In the direct gene transfer methods, the foreign gene of interest is delivered into the host plant cell without the help of a vector. The methods used for direct gene transfer in plants are:
Chemical mediated gene transfer e.g. chemicals like polyethylene glycol (PEG) and dextran sulphate induce DNA uptake into plant protoplasts.Calcium phosphate is also used to transfer
DNA into cultured cells.
Microinjection where the DNA is directly injected into plant protoplasts or cells (specifically into the nucleus or cytoplasm) using fine tipped (0.5 - 1.0 micrometerdiameter) glass needle or micropipette. This method of gene transfer is used to introduce DNA into large cells such as oocytes, eggs, and the cells of early embryo.
Electroporation involves a pulse of high voltage applied to protoplasts/cells/ tissues to make transient (temporary) pores in the plasma membrane which facilitates the uptake of foreign
DNA.
The cells are placed in a solution containing DNA and subjected to electrical shocks to cause holes in the membranes. The foreign DNA fragments enter through the holes into the cytoplasm and then to nucleus.
Particle gun/Particle bombardment - In this method, the foreign DNA containing the genes to be transferred is coated onto the surface of minute gold or tungsten particles (1-3 micrometers) and bombarded onto the target tissue or cells using a particle gun (also called as gene gun/shot gun/microprojectile gun).The microprojectile bombardment method was initially named as biolistics by its inventor Sanford (1988). Two types of plant tissue are commonly used for particle bombardment- Primary explants and the proliferating embryonic tissues.
Transformation - This method is used for introducing foreign DNA into bacterial cells e.g. E.
Coli. The transformation frequency (the fraction of cell population that can be transferred) is very good in this method. E.g. the uptake of plasmid DNA by E. coli is carried out in ice cold
CaCl2 (0-50C) followed by heat shock treatment at 37-450C for about 90 sec. The transformation efficiency refers to the number of transformants per microgram of added DNA.
The CaCl2 breaks the cell wall at certain regions and binds the DNA to the cell surface.
Conjuction - It is a natural microbial recombination process and is used as a method for gene transfer. In conjuction, two live bacteria come together and the single stranded DNA is transferred via cytoplasmic bridges from the donor bacteria to the recipient bacteria.
Liposome mediated gene transfer or Lipofection - Liposomes are circular lipid molecules with an aqueous interior that can carry nucleic acids. Liposomes encapsulate the DNA fragments and then adher to the cell membranes and fuse with them to transfer DNA fragments. Thus, the DNA enters the cell and then to the nucleus. Lipofection is a very efficient technique used to transfer genes in bacterial, animal and plant cells.
Selection of transformed cells from untransformed cells
The selection of transformed plant cells from untransformed cells is an important step in the plant genetic engineering. For this, a marker gene (e.g. for antibiotic resistance) is introduced into the plant along with the transgene followed by the selection of an appropriate selection medium (containing the antibiotic). The segregation and stability of the transgene integration and expression in the subsequent generations can be studied by genetic and molecular analyses
(Northern, Southern, Western blot, PCR).
3. Describe the features of Ti and Ri plasmids
Among Higher plants, Ti plasmid of Agrobacterium tumefaciens or Ri plasmid of rhizogenes is the best known vector. T- DNA, from Ti or Ri plasmid Agrobacterium, is considered to be a very potential vector for cloning experiments with higher plants. This will involve the following steps
(i)foreign DNA has to be first cloned into T-DNA of Ti or Ri plasmid
(ii) modified hybrid T-DNA can be transferred to the genome of plan cells by Agrobacterium infection. Recombinant Ti plasmids can also b obtained by cloning of a large fragment of Ti plasmid in pBR322. The recombinant Ti plasmids can then be used for transformation of higher plants.
This system can be widely used, since Agrobacterium infects nearly all dicotyledonous plants.
Such cloning in plants has proved to be of immense use to modify agricultural plants to increase the productivity.
Structure and Functions of Ti and Ri Plasmids - The most commonly used vectors for gene transfer in higher plants are based on tumour inducing mechanism of the soil bacterium
Agrobacterium tumefaciens, which is the causal organism for crown gall disease, A closely
related species A. rhizogenes causes hairy root disease. An understanding of the molecular basis of these diseases led to the utilization of these bacteria for developing gene transfer systems. It has been shown that the disease is caused due to the transfer of a DNA segment from the bacterium to the plant nuclear genome.
The DNA segment, which is transferred is called T - DNA and is part of a large Ti (tumour inducing) plasmid found in virulent strains of Agrobacterium tumefaciens. Similarly Ri (root inducing) megaplasmids are found in the virulent strains of A. rhizogenes. The Ti and Ri plasmids, inducing crown gall disease and hairy root disease respectively have been studied in great detail during the last decade. However, we will discuss only those aspects of these plasmids which are relevant to the design of vectors for gene transfer in higher plants.
Most Ti plasmids have four regions in common,
(i) Region A, comprising T-DNA is responsible for tumour induction, so that mutations in this region lead to the production of tumours with altered morphology (shooty or rooty mutant galls).
Sequences homologous to this region are always transferred to plant nuclear genome, so that the region is described as T-DNA (transferred DNA).
(ii) Region B is responsible for replication.
(iii) Region C is responsible for conjugation.
(iv) Region D is responsible for virulence, so that mutation in this region abolishes virulence.
This region is therefore called virulene
(v) region and plays a crucial role in the transfer of T-DNA into the plant nuclear genome. The components of this Ti plasmid have been used for developing efficient plant transformation vectors.
The T-DNA consists of the following regions:
(i) An one region consisting of three genes (two genes tms and tms2 representing 'shooty locus' and one gene tmr representing 'rooty locus') responsible for the biosynthesis of two phytohormones, namely indole acetic acid or lAA (an auxin) and isopentyladenosine 5'monophosphate (a cytokinin). These genes encode the enzymes responsible for the synthesis of these phytohormones, so that the incorporation of these genes in plant nuclear genome leads to the synthesis of these phytohormones in the host plant. The phytohormones in their turn alter the developmental programme, leading to the formation of crown gall
(ii) An os region responsible for the synthesis of unusual amino acid or sugar derivatives, which are collectively given the name opines. Opines are derived from a variety of compounds (e.g. arginine + pyruvate), that are found in plant cells. Two most common opines are octopine and
nopaline. For the synthesis of octopine and nopaline, the corresponding enzymes octopine synthase and nopaline synthase are coded by T- DNA.
Depending upon whether the Ti plasmid encodes octopine or nopaline, it is described as octopine-type Ti plasmid or nopalinetype Ti plasmid. Many organisms including higher plants are incapable of utilizing opines, which can be effectively utilized by Agrobacterium. Outside the T-DNA region, Ti plasmid carries genes that, catabolize the opines, which are utilized as a source of carbon and nitrogen.
The T-DNA regions on all Ti and Ri plasmids are flanked by almost perfect 25bp direct repeat sequences, which are essential for T-DNA transfer, acting only in cis orientation. It has also been shown that any DNA sequence, flanked by these 25bp repeat sequences in the correct orientation, can be transferred to plant cells, an attribute that has been successfully utilized for
Agrobacterium mediated gene transfer in higher plants leading to the production of transgenic plants.
Besides 25bp flanking border sequences (with T DNA), vir region is also essential for T-DNA transfer. While border sequences function in cis orientation with respect to T -DNA, vir region is capable of functioning even in trans orientation. Consequently physical separation of T-DNA and vir region onto two different plasmids does not affect T-DNA transfer, provided both the plasmids are present in the same Agrobacterium cell. This property played an important role in designing the vectors for gene transfer in higher plants, as will be discussed later.
The vir region (approx 35 kbp) is organized into six operons, namely vir A, vir B, vir C, vir D, vir E, and vir G, of which four operons (except vir A and vir G) are polycistronic. Genes vir A,
B, D, and G are absolutely required for virulence; the remaining two genes vir C and E are required for tumour formation. The vir A locus is expressed constitutively under all conditions.
The vir G locus is expressed at low levels in vegetative cells, but is rapidly induced to higher expression levels by exudates from wounded plant tissue. The vir A and vir G gene products regulate the expression of other vir loci. The vir A product (Vir A) is located on the inner membrane of Agrobacterium cells and is probably a chemoreceptor, which senses the presence of phenolic compounds (found in exudates of wounded plant tissue), such as acetosyringone and
β-hydroxyaceto syringone.
Signal transduction proceeds via activation (possibly phosphorylation) of Vir G (product of gene vir G), which in its turn induces expression of other vir genes.
4. What are 35S promoters? Explain with diagram.
Analysis on the CaMV 35 promoter is divided into a discussion of:
•the promoter itself
•sequences identified in patents as "35S enhancer regions"
•the "minimal" promoter
At the beginning of the 1980s, Chua and collaborators at the Rockefeller University isolated the promoter responsible for the transcription of the whole genome of a Cauliflower mosaic virus
(CaMV) infecting turnips. The promoter was named CaMV 35S promoter ("35S promoter") because the coefficient of sedimentation of the viral transcript whose expression is naturally driven by this promoter is 35S. It is one of the most widely used, general-purpose constitutive promoters.
The 35S promoter is a very strong constitutive promoter, causing high levels of gene expression in dicot plants. However, it is less effective in monocots, especially in cereals. The differences in behavior are probably due to differences in quality and/or quantity of regulatory factors. The promoter responsible for the transcription of another part of the genome of CaMV, the CaMV
19S promoter, is also used as a constitutive promoter, but is not as widely used as the 35S promoter.
Part – C
1. Elaborate on antisense RNA technology and emphasis its role in fruit ripening.
Antisense RNA is a single-stranded RNA that is complementary to a messenger RNA (mRNA) strand transcribed within a cell. Antisense RNA may be introduced into a cell to inhibit translation of a complementary mRNA by base pairing to it and physically obstructing the translation machinery. This effect is therefore stoichiometric. An example of naturally occurring mRNA antisense mechanism is the hok/sok system of the E.coli R1 plasmid. Antisense RNA has long been thought of as a promising technique for disease therapy; the only such case to have reached the market is the drug fomivirsen. One commentator has characterized antisense RNA as one of "dozens of technologies that are gorgeous in concept, but exasperating in
[commercialization]". Generally, antisense RNA still lack effective design, biological activity, and efficient route of administration.
Historically, the effects of antisense RNA have often been confused with the effects of RNA interference (RNAi), a related process in which double-stranded RNA fragments called small interfering RNAs trigger catalytically mediated gene silencing, most typically by targeting the
RNA-induced silencing complex (RISC) to bind to and degrade the mRNA. Attempts to
genetically engineer transgenic plants to express antisense RNA instead activate the RNAi pathway, although the processes result in differing magnitudes of the same downstream effect, gene silencing. Well-known examples include the Flavr Savr tomato and two cultivars of ringspot-resistant papaya.
Transcription of longer cis-antisense transcripts is a common phenomenon in the mammalian transcriptome. Although the function of some cases have been described, such as the Zeb2/Sip1 antisense RNA, no general function has been elucidated. In the case of Zeb2/Sip1, the antisense noncoding RNA is opposite the 5' splice site of an intron in the 5'UTR of the Zeb2 mRNA.
Expression of the antisense ncRNA prevents splicing of an intron that contains a ribosome entry site necessary for efficient expression of the Zeb2 protein. Transcription of long antisense ncRNAs is often concordant with the associated protein-coding gene, but more detailed studies have revealed that the relative expression patterns of the mRNA and antisense ncRNA are complex
Narrate the process of T-DNA transfer during agrobacterium mediated transformation 2.
The transferring vehicle to the plant nucleus is a ssT-DNA-protein complex. Is must be translocated to the plant nucleus passing through three membranes, the plant cell wall and cellular spaces. According to the most accepted model, the ssT-DNA-VirD2 complex is coated by the 69 kDa VirE2 protein, a single strand DNA binding protein. This cooperative association prevents the attack of nucleases and, in addition, extends the ssT-DNA strand reducing the complex diameter to approximately 2 nm, making the translocation through membrane channels easier. However, that association does not stabilizes T-DNA complex inside Agrobacterium
(Zupan et al., 1996). VirE2 contains two plant nuclear location signals (NLS) and VirD2 one
(Bravo Angel et al, 1998). This fact indicates that both proteins presumably play important roles once the complex is in the plant cell mediating the complex uptake to the nucleus (Herrera-
Estrella et al., 1990; Shurvinton et al., 1992; Rossi et al., 1993, Tinland et al., 1995, Zupan et al.,
1996). The deletion of NLS in one of these proteins reduces, but does not totally inhibit, the ssT-
DNA transfer and its integration into plant genome, evidencing the other partner can at least partially assume the function of the absent protein.
It is known that VirE1 is essential for the export of VirE2 to the plant cell, although other specific functions are still uncharacterized (Binns et al., 1995). Bacterial strains mutated in virE1, cannot export VirE2 which is accumulated inside the bacterium. Such mutants can be complemented if coinfected with a strain that can export VirE2, indicating that this protein can
be exported independently and that the transfer of VirE2 as part of the ss-T-DNA complex is not necessary for the transmission event (Sundberg et al., 1996) being possible to transfer naked T-
DNA to the plant cell (Binns et al., 1995; Sundberg et al., 1996).
From these experimental evidences, an alternative model was brought to light for ssT-DNA complex transfer. This model proposes that the transfer complex is a single-strand DNA covalently bound at its 5'-end with VirD2, but uncoated by VirE2. The independent export of
VirE2 to plant cell is presented as a natural process, and once the naked ssT-DNA-VirD2 complex is inside the plant cell, it is coated by VirE2 (Binns et al., 1995; Lessl et al., 1994). It is also possible that the process can be performed by one of the proposed alternatives ways according to the infection conditions.
Previous researches described the role of 9.5 kb virB operon in the generation of a suitable cell surface structure for the ssT-DNA complex transfer from bacterium to plant
(Finberg et al., 1995; Stephens et al., 1995; Zhou and Christie, 1997; Dang and Christie, 1997;
Rashkova et al., 1997, Fernandez et al., 1996; Beaupré et al., 1997). The VirD4 protein is also required for the ss-T-DNA transport. The function of VirD4 is the ATP-dependent linkage of protein complex necessary for T-DNA translocation (Firth et al., 1996).
VirB are proteins that presents hydrophathy characteristics similar to other membraneassociated proteins (Kuldau et al., 1990; Shirasu et al., 1990, 1994; Thompson et al., 1988; Ward et al., 1988). VirD4 is a transmembrane protein but predominantly located at the cytoplasmatic side of the cytoplasmic membrane (Okamoto et al., 1991). Comparative studies showed a high degree of homology between the virB operon and transfer regions of broad host range (BHR) plasmids in genetic organization, nucleotide sequence and protein function (Pohlman et al.,
1994; Lessl et al., 1992). Both systems deliver non-self transmissible DNA-protein complex to recipient host cell. In addition, they have the capacity to DNA interkingdom delivery
(Heinemann and Sprague, 1989; Bundock et al., 1995; Piers et al., 1996) suggesting that the T-
DNA transfer apparatus and conjugation systems are related and probably evolved from a common ancestral (Christie et al., 1997; Oger et al., 1998).
The majority of VirB proteins are assembled as a membrane-spanning protein channel involving both membranes (Shirasu and Kado, 1993a, 1993b; Shirasu et al., 1994; Stephens et al., 1995; Das and Xie, 1998). Except for VirB11, they have multiple periplasmic domains
(Christie, 1997). VirB1 is the only member of VirB proteins found in the extracellular milieu
(Baron et al., 1997) although it is possible that some of the other VirB proteins may be redistributed during the process of biogenesis and functioning of the transcellular conjugal channel (Christie, 1997). That could be the case of the VirB2, a protein with deduced
extracellular functions. Vir B2 is translated as a 12 kDa proprotein, which is later processed by proteolysis to its mature 7 kDa functional form (Jones et al., 1996).
VirB4 and VirB11 are hydrophilic ATPases necessary for active DNA transfer. Vir B11 lacks continuous sequence of hydrophobic residues, formiing periplasmic domains. Despite these structural characteristics, less than one third of VirB11 constitutes its soluble fraction, while the rest of the protein remains associated with the cytoplasmic membrane (Rashkova et al., 1997).
These characteristics are atypical for this type of protein and evidence the possible dynamic coexistence of different conformational forms in vivo . VirB4 tightly associates with the cytoplasmic membrane (Dang and Christie, 1997). It contains two putative extracellular domains conferring transmembrane topology to this protein, which presumably allows the ATP-dependent conformational change in the conjugation channel. Probably, the functional forms of VirB4 and
VirB11 are homo- and heterodimers (Dang and Christie, 1997). The VirB4 synthesis is well correlated with the accumulation and distribution of VirB3. Other protein, VirB7, seems to be crucial for the conformation of the transfer apparatus. VirB7 interacts with VirB9 forming heterodimers and probably higher-order multimeric complexes. The synthesis of VirB9 and its stable accumulation depends of heterodimer conformation, indicating that VirB9 alone may be unstable and requires the association with VirB7 (Anderson et al., 1996). In this intermolecular conformation the monomeric subunits are joined by disulfide bridges. The VirB7-VirB9 heterodimer is assumed to stabilize other Vir proteins during assembly of functional transmembrane channels (Fernandez et al., 1996; Spudich et al., 1996).
Some of the initial steps of biogenesis of ssT-DNA complex apparatus. have been recently identified. Firstly, VirB7 and VirB9 monomers are exported to the membrane and processed.
They interact each other to form covalently cross-linked homo- and heterodimers. Although the role of both types of dimers in the biogenesis of the transfer apparatus is widely accepted, it is likely that only heterodimers are essential (Fernández et al., 1996; Spudich et al., 1996).
Subsequently the VirB7-VirB9 heterodimer is sorted to the outer membrane. The sorting mechanism has not been elucidated (Christie, 1997) the next step implies the interaction with the other Vir proteins for assembling the transfer channel with the contribution of the transglycosidase VirB1.
It is known that VirB2 through VirB11 are essential for DNA transfer, suggesting that these proteins are fundamental component of the transfer apparatus (Berger and Christie, 1993,
1994) while VirB1 has a lesser contribution to this process.
Two accessory vir operons, present in the octopine Ti-plasmid, are virF and virH. The virF operon encodes for a 23 kDa protein that functions once the T-DNA complex is inside the plant
cells via the conjugal channel or independently, as it was assumed for VirE2 export. The role of
VirF seems to be related with the nuclear targeting of the ssT-DNA complex but its contribution is less important than in the case of VirF (Hooykass and Schilperoort, 1992). The virH operon consists of two genes that code for VirH1 and VirH2 proteins. These Vir proteins are not essential but could enhance the transfer efficiency, detoxifying certain plant compounds that can affect the bacterial growth (Kanemoto et al., 1989). If that is the function of VirH proteins, they play a role in the host range specificity of bacterial strain for different plant species.
Integration of T-DNA into plant genome
Inside the plant cell, the ssT-DNA complex is targeted to the nucleus crossing the nuclear membrane. Two Vir proteins have been found to be important in this step: VirD2 and VirE2, which are the most important, and probably VirF, which has a minor contribution to this process
(Hooykaas and Schilperoort, 1992). The nuclear location signals (NLS) of VirD2 and VirE2 play an important role in nuclear targeting of the delivered ss-T-DNA complex, as early described.
VirD2 has one functional NLS. The ssT-DNA complex is a large (up to 20 kb) nucleoprotein complex containing only one 5'end covalently attached VirD2 protein per complex. But the complex is coated by a large number of VirE2 molecules (approximately 600 per a 20 kb T-
DNA), and each of them has two NLS. The two NLS of VirE2 have been considered important for the continuos nuclear import of ss-T-DNA complex, probably by keeping both sides of nuclear pores simultaneously open. The nuclear import is probably mediated also by specific
NLS-binding proteins, which are present in plant cytoplasm.
The final step of T-DNA transfer is its integration into the plant genome. The mechanism involved in the T-DNA integration has not been characterized. It is considered that the integration occurs by illegitimate recombination (Gheysen et al., 1991, Lehman et al., 1994;
Puchta, 1998). According to this model, paring of a few bases, known as micro-homologies, are required for a pre-annealing step between T-DNA strand coupled with VirD2 and plant DNA.
These homologies are very low and provide jus a minimum specificity for the recombination process by positioning VirD2 for the ligation. The 3´-end or adjacent sequences of T-DNA find some low homologies with plant DNA resulting in the first contact (synapses) between the Tstrand and plant DNA and forming a gap in 3'-5' strand of plant DNA. Displaced plant DNA is subsequently cut at the 3'-end position of the gap by endonucleases, and the first nucleotide of the 5' attaches to VirD2 pairs with a nucleotide in the top (5'-3') plant DNA strand. The 3' overhanging part of T-DNA together with displaced plant DNA are digested away, either by endonucleases or by 3'-5' exonucleases. Then, the 5' attached to VirD2 end and other 3'-end of Tstrand (paired with plant DNA during since the first step of integration process) joins the nicks in the bottom plant DNA strand. Once the introduction of T-strand in the 3'-5' strand of the plant
DNA is completed, a torsion followed by a nick into the opposite plant DNA strand is produced.
This situation activates the repair mechanism of the plant cell and the complementary strand is synthesized using the early inserted T-DNA strand as a template (Tinland et al., 1995).
VirD2 has an active role in the precise integration on T-strand in the plant chromosome. The release of VirD2 protein may provide the energy containing in its phosphodiester bond, at the
Tyr29 residue, with the first nucleotide of T-strand, providing the 5'-end of the T-strand for ligation to the plant DNA. This phosphodiester bond can serve as electrophilic substrate for nucleophilic 3'-OH from nicked plant DNA. (Jayaram, 1994). When the mutant VirD2 protein is transferred attached to the T-strand, the integration process take place with the loss of nucleotides at the 5'-end of the T-strand (Tinland et al., 1995).
3. What are the methods of gene transfer in plants?
In conventional breeding, the pool of available genes and the traits they code for is limited due to sexual incompatibility to other lines of the crop in question and to their wild relatives. This restriction can be overcome by using the methods of genetic engineering, which in principle allow introducing valuable traits coded for by specific genes of any organism (other plants, bacteria, fungi, animals, viruses) into the genome of any plant. The first gene transfer experiments with plants took place in the early 1980s. Normally, transgenes are inserted into the nuclear genome of a plant cell. Recently it has become possible to introduce genes into the genome of chloroplasts and other plastids (small organelles of plant cells which possess a separate genome).
Transgenic plants have been obtained using Agrobacterium-mediated DNA-transfer and direct
DNA-transfer, the latter including methods such as particle bombardment, electroporation and polyethylenglycol permeabilisation. The majority of plants have been transformed using
Agrobacterium mediated transformation.
Agrobacterium-mediated Gene Transfer :
The Agrobacterium-mediated technique involves the natural gene transfer system resident in the bacterial plant pathogens of the genus Agrobacterium. In nature, Agrobacterium tumefaciens and
Agrobacterium rhizogenes are the causative agents of the crown gall and the hairy root diseases, respectively. The utility of Agrobacterium as a gene transfer system was first recognized when it was demonstrated that these plant diseases were actually produced as a result of the transfer and integration of genes from the bacteria into the genome of the plant. Both Agrobacterium species carry a large plasmid (small circular DNA molecule) called Ti in A. tumefaciens and Ri in A.
rhizogenes. A segment of this plasmid, designated T-(for transfer) DNA, is transmitted by this organism into individual plant cells, usually within wounded tissue (see figure 2.1). The T-DNA segment penetrates the plant cell nucleus and integrates randomly into the genome where it is stably incorporated and inherited like any other plant gene in a predictable, dominant Mendelian fashion. Expression of the natural genes on the T-DNA results in the synthesis of gene products that direct the observed morphological changes, i. e. tumor or hairy root formation.
In genetic engineering, the tumor inducing genes within the T-DNA which cause the plant disease are removed and replaced by foreign genes. These genes are then stably integrated into the genome of the plant after infection with the altered strain of Agrobacterium, just like the natural T-DNA. Because all tumor-inducing genes are removed, the gene transfer does not induce any disease symptoms. This reliable method of gene transfer is well suited for plants which are susceptible to infection by Agrobacterium. Unfortunately, many species, especially economically important legumes and monocotyledons such as cereals, do not respond positively to Agrobacterium-mediated transformation. For these plants, the following methods of direct
DNA uptake must be applied.
Particle Bombardment :
This method, also referred to as biolistic transformation (from biological ballistics) involves coating biologically active DNA onto small tungsten or gold particles (1-5 ?m in diameter) and accelerating them into plant tissue at high velocity. The particles penetrate the plant cell wall and lodge themselves within the cell where the DNA is liberated resulting in transformation of the individual plant cell in an explant. This technique is generally less efficient than Agrobacteriummediated transformation, but has nevertheless been particularly useful in several plant species, most notably in cereal crops. The introduction of DNA into organized, morphogenic tissues such as seeds, embryos or meristems has enabled the successful transformation and regeneration of rice, wheat, soybean and maize, thus demonstrating the enormous potential of this method .
Electroporation and Direct DNA Entry into Protoplasts
Electroporation is a process whereby very short pulses of electricity are used to reversibly permeabilize lipid bilayers of plant cell membranes. The electrical discharge enables the diffusion of macromolecules such as DNA through an otherwise impermable plasma membrane.
Because the plant cell wall will not allow the efficient diffusion of many transgene constructs, protoplasts (cells without cell walls) must be prepared. This requirement presents a major obstacle for many applications as protocols making possible the regeneration of protoplasts into complete plants do not exist for many species.
DNA uptake by plant protoplasts can also be stimulated by phosphate or calcium/polyethylene glycol (PEG) coprecipitation. However, these methods all suffer from the drawback that they use protoplasts as the recipient host which often cannot be regenerated into whole plants.
Transgene Expression
In most cases, the introduction of a gene into the plant genome will only have an effect on the plant if the transgene is expressed, i. e. transcribed into mRNA and translated into a protein. A promoter is a sequence of nucleic acids where the RNA polymerase (a complex enzyme synthesizing the mRNA transcript) attaches to the DNA template. The nature of the promoter defines (together with other expression-regulating elements), under which conditions and with which intensity a gene will be transcribed. The promoter of the 35S gene of cauliflower mosaic virus is used very frequently in plant genetic engineering. This promoter confers high-level expression of exogenous genes in most cell types from virtually all species tested. As it is often advantageous to express a transgene only in certain tissues or quantities or at certain times, a number of other promoters are available, e.g. promoters inducing gene expression after wounding or during fruit ripening only.
Methods of gene transfer currently employed result in the random integration of foreign DNA throughout the genome of recipient cells. The site of insertion may have a strong influence on the expression levels of the exogenous gene, resulting in different expression levels of an introduced gene, even if the same promoter/gene construct was used. The exact mechanism of this phenomenon are not yet fully understood.
Selection and Plant Regeneration
In a transformation experiment, the proportion of transformed cells is usually small compared to the number of cells which remain unaltered. In order to select only cells which have actually incorporated the new genes, the genes coding for the desired trait are fused to a gene which allows selection of transformed cells, so-called marker genes. The expression of the marker gene enables the transgenic cells to grow in presence of a selective agent, usually an antibiotic or a herbicide, while cells without the marker gene die. One of the most commonly used marker genes is the bacterial aminoglycoside-3' phosphotransferase gene (APH(3')II), also referred to as neomycin phosphotransferase 11 (NPTII). This gene codes for an enzyme which inactivates the antibiotics kanamycin, neomycin and G418 through phosphorylation. In addition to NPTII, a number of other antibiotic resistance genes have been used as selective markers, e.g. hygromycin phosphotransferase gene conferring resistance to hygromycin.
Another group of selective markers are herbicide tolerance genes. Herbicide tolerance has been obtained through the incorporation and expression of a gene which either detoxifies the herbicide in a similar manner as the antibiotic resistance gene products or a gene that expresses a product which acts like the herbicide target but is not affected by the herbicide. Herbicide tolerance may not only serve as a trait useful for selection in the development of transgenic plants, but also has some commercial interest. Herbicide tolerance transgenic plants are therefore among the first crops approaching market introduction.
Transformation of plant protoplasts, cells and tissues is usually only useful if the they can be regenerated into whole plants. The rates of regeneration vary greatly not only among different species, but also between cultivars of the same species. As mentioned in capter 2.2, in many cases regeneration of whole plants from cells is not possible or very difficult. Besides the ability to introduce a gene into the genome of a plant species, regeneration of intact, fertile plants out of transformed cells or tissues is the most limiting step in developing transgenic plants.
4. Explain Shikimate pathway and its role in the production of secondary metabolites.
Shikimic acid, more commonly known as its anionic form shikimate, is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi (Illicium anisatum), from which it was first isolated.
Shikimic acid is a precursor for: the aromatic amino acids phenylalanine and tyrosine, indole, indole derivatives and aromatic amino acid tryptophan, many alkaloids and other aromatic metabolites, tannins, flavonoids, and lignin.
In the pharmaceutical industry, shikimic acid from the Chinese star anise is used as a base material for production of oseltamivir (Tamiflu). Although shikimic acid is present in most autotrophic organisms, it is a biosynthetic intermediate and generally found in very low concentrations. The low isolation yield of shikimic acid from the Chinese star anise is blamed for the 2005 shortage of oseltamivir. Shikimic acid can also be extracted from the seeds of the sweetgum fruit, which is abundant in North America, in yields of around 1.5%. 4 kg of sweetgum seeds is needed for fourteen packages of Tamiflu. By comparison, star anise has been reported to yield 3 to 7% shikimic acid. Recently biosynthetic pathways in E. coli have been enhanced to allow the organism to accumulate enough material to be used commercially.
Unit III
Animal cell cultures: Culture media – composition and preparation, Balanced salt solution and simple growth medium, chemical, physical and metabolic functions of different constituents of culture medium-Role of CO2, serum and protein-free defined media and their applications;
Culturing and maintenance of different animal cell lines (Primary and established cell lines).
Characterization of cultured cell, measurement of viability, cyto-toxicity and growth parameters.
Stem cell cultures, embryonic stem cell and their applications, cell culture based vaccines, measurement of cell death, apoptosis, scaling up animals cell cultures.
Part – A
1.
Animal cell culture
It can be described as in vitro maintenance and propagation of animal Cells using suitable nutrient media. Animal cell culture is the complex process by which cells are grown under controlled conditions. In practice, the term "cell culture" has come to refer to the culturing of cells derived from multicellular eukaryotes, especially animal cells.
2.
Media
Any liquid or solid preparation made specifically for the growth, storage, or transport of of cells.
3.
Serum
Blood serum, a component of blood which is collected after coagulation.
4.
Buffer
Buffer solution, a solution which reduces the change of pH upon addition of small amounts of acid or base, or upon dilution eg HEPES
5.
HEPES
It ( 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid ) is a zwitterionic organic chemical buffering agent; one of the twelve Good's buffers. HEPES is widely used in cell culture, largely because it is better at maintaining physiological pH despite changes in carbon dioxide concentration (produced by cellular respiration) when compared to bicarbonate buffers, which are also commonly used in cell culture.
6.
What is the role of Co
2
in cell culture
Carbon di oxide influences the pH of the media.
Buffering action of bicarbonate helps maintain PH.
HEPES containing media well regulates ph.
7.
Adhersion
•
Majority Of Cells Adhere On Plastic (Treated) Provided They Are Not Transformed
•
It Was Observed That Cells Prefer –vely Charged Glass Surface
•
Plastic (polystyrene) Is Tissue Culture Treated
–
With High Energy Ionizing Radiation
– Electric Ion Discharge
•
Adhesion Is Mediated By Surface Receptors And Matrix
–
Matrix Is Secreted By Cells, Adheres To Charged Plastic
–
Receptors Bind to Matrix
8.
What are the classes of Cell Surface Adhesion Molecules
Three Major Classes
•
Cell-Cell Adhesion Molecules
•
Cell-Substrate Molecules
•
Proteoglycans
9.
Cell cycle
•
4 Phases
– M Phase, mitosis occurs
•
Chromatin condensation, sister chromatid separation
•
Daughter cells
– G1 Phase
•
Progression to DNA SYNTHESIS
•
Alternatively Go OR differentiation
• Restriction Points
–
S Phase
•
DNA Synthesis
•
Progression to G2
– G2 Phase
•
Integrity of DNA Checkpoints
•
Apoptosis is an option
– DNA fragmentation, cell shrinkage, formation of small vesicles
10.
Dedifferentiation
•
Inability To Express In Vivo Phenotype Is Attributed To Dedifferentiation
• Still Not Clear If Dedifferentiation Occurs
–
Wrong lineage expansion is a possibility
–
Undifferentiated cells dominate
–
Absence of appropriate inducers, hormones, matrix
•
Deadaptation vs Dedifferentiation
– Deadaptation-enviroment suppresses phenotype, reversible
–
Dedifferentiation-conversion to primitive phenotype, irreversible
11.
Cell synchronization
Its a process by which cells at different stages of the cell cycle in a culture are brought to the same phase. "Cell synchrony" is required to study the progression of cells through the cell cycle.
The types of synchronizations are broadly categorized into two groups: "Physical Fractionation" and "Chemical Blockade."
12.
Senescence
Senescence or biological aging is the change in the biology of an organism as it ages after its maturity. Such changes range from those affecting its cells and their function to that of the whole organism. The word senescence is derived from the Latin word senex , meaning old man , old age , or advanced in age .
13.
Apoptosis
Its the process of programmed cell death (PCD) that may occur in multicellular organisms.
14.
Types of cell culture
Primary, secondary cell culture, organ culture, stem cell culture & cell lines.
Based on the origin of the cells
Primary culture (directly from animal or plant tissue)
Extended culture (multipassage culture) – cell strain
Established (transformed) cell lines
15.
Primary cell culture
A culture derived directly from a tissue, cells organ from an organism and before first sub culturing.
16.
Disaggregation
Mononuclear cells can be released from soft tissues by enzymatic digestion with enzymes such as collagenase , trypsin , or pronase , which break down the extracellular matrix .
17.
Passaging or subculturing
Passaging (also known as subculture or splitting cells) involves transferring a small number of cells into a new vessel, usually done by disaggregation using enzyme trypsin. Cells can be cultured for a longer time if they are split regularly, as it avoids the senescence associated with prolonged high cell density.
18.
Secondary cell culture
Its the culture derived from primary cell culture i.e., after first sub culturing.
The cells in a primary culture multiply and form a confluent layer. When subculturing is done from the primary cell culture it is called secondary cell culture.
19.
Suspension culture
A culture in which cells will multiply when suspended in growth medium.
20.
Cell line & Cell strain
Cell line: A propagated culture after first subculture. Cell strain: a characterized cell line derived by selection or cloning.
21.
Continuous cell line
Cell line or cell strain having the capacity for infinite survival. Previously known as established and often referred as immortal.
22.
Trans-differentiation
Cells from one lineage acquiring the ability to differentiate into cells of a different lineage.
23.
Stem cell
Stem cells are biological cells found in all multicellular organisms, that can divide through mitosis and differentiate into diverse specialized cell types and can self renew to produce more stem cells.
24.
Totipotent (a.k.a omnipotent) stem cells
They can differentiate into embryonic and extraembryonic cell types. Such cells can construct a complete, viable organism. These cells are produced from the fusion of an egg and sperm cell.
Cells produced by the first few divisions of the fertilized egg are also totipotent.
25.
Pluripotent stem cells
They are the descendants of totipotent cells and can differentiate into nearly all cells, i.e. cells derived from any of the three germ layers .
26.
Multipotent stem cells
They can differentiate into a number of cells, but only those of a closely related family of cells.
27.
Oligopotent stem cells
They can differentiate into only a few cells, such as lymphoid or myeloid stem cells.
28.
Unipotent cells
They can produce only one cell type, their own, but have the property of self-renewal which distinguishes them from non-stem cells (e.g. muscle stem cells).
29.
Cell viability assays
Cell viability measurements assess healthy cells in a sample. This can be accomplished either by directly counting the number of healthy cells or by measuring an indicator for healthy cells in cell populations (e.g., in a microplate assay). Whether the cells are actively dividing or quiescent is not distinguished. An increase in cell viability indicates cell growth, while a decrease in viability can be interpreted as the result of either toxic effects of compounds/agents or suboptimal culture conditions.
30.
Cyto-toxicity & Cyto-toxicity assays
Cytotoxicity is the quality of being toxic to cells . Cytotoxicity assays are used to measure the toxicity of a drug on cells. Eg. MTT & SRB assays.
31.
Organ culture
The maintenance or growth of organ primodia or the whole or parts of an organ in vitro in a way that may allow differentiation and preservation of the architecture or function of the organs.
32.
Cryopreservation
Its a process where cells or whole tissues are preserved by cooling to low sub-zero temperatures, such as (typically) 77 K or −196 °C (the boiling point of liquid nitrogen). At these low temperatures, any biological activity, including the biochemical reactions that would lead to cell death, is effectively stopped.
33.
Thawing
After cryopreservation thawing (defrost) is done when we need the preserved cell lines for use.
Defrost the vial in a 37°C water bath with constant, moderate agitation, until ice in the ampule is no longer visible.
34.
Insect cell line characters
Sf9 and Sf21 cell lines are the traditional cell lines used with baculovirus and originated at the
USDA Insect Pathology Laboratory. The cell lines are also suitable for use in the InsectSelect
TM
System. These two cell lines originated from the IPLBSF-21 cell line, derived from the pupal ovarian tissue of the fall army worm, Spodoptera frugiperda .
Characteristics: Sf9 and Sf21 share the following characteristics:
– Grow well in monolayer and suspension culture
–
Adaptable to serum-free medium
Part – B
1.
Write a note on Animal cell culture
Its the process of culture of animal cells outside the tissue ( ex vivo ) from which they were obtained. The process of ACC is carried out under strict laboratory conditions of asepsis, sterility and controlled environment involving temperature, gases and pressure. It should mimic the in vivo environment successfully such that the cells are capable of survival and proliferation in a controlled manner.
Epithelial cells in culture, stained for keratin (red) and DNA (green)
Cell culture is the process by which prokaryotic or eukaryotic cells are grown under controlled conditions. In practice the term "cell culture" has come to refer to the culturing of cells derived from multicellular eukaryotes, especially animal cells. The historical development and methods of cell culture are closely interrelated to those of tissue culture and organ culture. Animal cell culture became a common laboratory technique in the 1950s, but the concept of maintaining live cell lines separated from their original tissue source was discovered in the 19th century.
2.
Write a note on tissue culture
The term tissue culture refers to the culture of whole organs, tissue fragments as well as dispersed cells on a suitable nutrient medium. It can be divided into
(1) organ culture and
(2) cell culture mainly on the basis of whether the tissue organisation is retained or not.
In organ cultures, whole embryonic organs or small tissue fragments are cultured in vitro in such a manner that they retain their tissue architecture. In contrast, cell cultures are obtained either by
enzymatic or mechanical dispersal of tissues into individual cells or by spontaneous migration of cells from explants; they are maintained as attached monolayers or as cell suspensions.
Freshly isolated cell cultures are called primary cultures; they are usually heterogeneous and slow growing, but are more representative of the tissue of their origin both in cell type and properties. Once a primary culture is subcultured, it gives rise to cell lines, which may either die after several subcultures (such cell lines are known as finite cell lines) or may continue to grow indefinitely (these are called continuous cell lines).
Usually, normal tissues give rise to finite cell lines, while tumours give rise to continuous cell lines. But there are several examples of continuous cell lines, which were derived from normal tissues and are themselves nontumorigenic, e.g., MDCK dog kidney, 3T3 fibroblasts, etc.
The evolution of continuous cell lines from primary cultures is supposed to involve a mutation, which alters their properties as compared to those of finite lines.
3.
Write a note on animal cell culture media
Culture Media - The nutrient media used for culture of animal cells and tissues must be able to support their survival as well as growth, i.e., must provide nutritional, hormonal and stromal factors.
The various types of media used for tissue culture may be grouped into two broad categories:
(1) natural media and
(2) artificial media.
The choice of medium depends mainly on the type of cells to be cultured (normal, immortalized or transformed), and the objective of culture (growth, survival, differentiation, production of desired proteins).
Nontransformed or normal cells (finite life span) and primary cultures from healthy tissues require defined quantities of proteins, growth factors and hormones even in the best media developed so far. But immortalized cells (spontaneously or by transfection with viral sequences) produce most of these factors, but may still need some of the growth factors present in the serum.
In contrast, transformed cells (autonomous growth control and malignant properties) synthesize their own growth factors; in fact, addition of growth factors may even be detrimental in such cases. But even these cultures may require factors like insulin, transferrin, silenite, lipids, etc
4.
Cell Surface Adhesion Molecules
• Three Major Classes
–
Cell-Cell Adhesion Molecules
•
CAMs (Ca
2+
Independent)
•
Cadherins (Ca
2+
Dependent)
•
Primarily Between Homologous Cells
•
Signaling occurs
–
Cell-Substrate Molecules
• Integrins
•
Bind to fibronectin, entactin, laminin, collagen
•
Bind the specific motif (RGD, arginine, glycine,aspratic)
•
Comprised of
and
unit
Proteoglycans
• Also Binds Matrix or Other Proteoglycans
•
Not Via RGD Motif
•
Low affinity Growth Factor Receptors
• May Aid Binding To Higher Affinity Receptors
•
No Signaling Capacity
5.
Write a note on cell senescence
Senescence or biological aging is the change in the biology of an organism as it ages after its maturity. Such changes range from those affecting its cells and their function to that of the whole organism. There are a number of theories as to why senescence occurs, including ones that claim it is programmed by gene expression changes and that it is the accumulative damage of biological processes. The word senescence is derived from the Latin word senex , meaning old man , old age , or advanced in age .
Cellular senescence
Cellular senescence (upper) Primary mouse embryonic fibroblast cells (MEFs) before senescence. Spindle-shaped. (lower) MEFs became senescent after passages. Cells grow larger, flatten shape and expressed senescence-associated β-galactosidase (SABG, blue areas), a marker of cellular senescence.
Cellular senescence is the phenomenon by which normal diploid cells lose the ability to divide, normally after about 50 cell divisions in vitro. Some cells become senescent after fewer replications cycles as a result of DNA double strand breaks, toxins, etc. This phenomenon is also known as "replicative senescence", the "Hayflick phenomenon", or the Hayflick limit in honour of Dr. Leonard Hayflick who was the first to publish this information in 1965. In response to
DNA damage (including shortened telomeres), cells either age or self-destruct (apoptosis, programmed cell death) if the damage cannot be easily repaired. In this 'cellular suicide', the death of one cell, or more, may benefit the organism as a whole. For example, in plants the death of the water-conducting xylem cells (tracheids and vessel elements) allows the cells to function more efficiently and so deliver water to the upper parts of a plant. The ones that do not selfdestruct remain until destroyed by outside forces.
Aging of the whole organism
Organismal senescence is the aging of whole organisms. In general, aging is characterized by the declining ability to respond to stress, increased homeostatic imbalance, and increased risk of aging-associated diseases. Death is the ultimate consequence of aging, though "old age" is not a scientifically recognized cause of death because there is always a specific proximal cause, such
as cancer, heart disease, or liver failure. Aging of whole organisms is therefore a complex process that can be defined as "a progressive deterioration of physiological function, an intrinsic age-related process of loss of viability and increase in vulnerability".
Differences in maximum life span among species correspond to different "rates of aging". For example, inherited differences in the rate of aging make a mouse elderly at 3 years and a human elderly at 80 years. These genetic differences affect a variety of physiological processes, including the efficiency of DNA repair, antioxidant enzymes, and rates of free radical production.
Supercentenarian Ann Pouder (8 April 1807 – 10 July 1917) photographed on her 110th birthday. A heavily lined face is common in human senescence.
Senescence of the organism gives rise to the Gompertz–Makeham law of mortality, which says that mortality rate rises rapidly with age.
Some animals, such as some reptiles and fish, age slowly (negligible senescence) and exhibit very long lifespans. Some even exhibit "negative senescence", in which mortality falls with age, in disagreement with the Gompertz–Makeham "law".
Whether replicative senescence (Hayflick limit) plays a causative role in organismal aging is at present an active area of investigation.
6.
Write a note on Apoptosis
Programmed cell death is called apoptosis . The pattern of events in death by suicide is so orderly that the process is often called programmed cell death or PCD . The cellular machinery of programmed cell death turns out to be as intrinsic to the cell as, say, mitosis.
Cells that are induced to commit suicide:
shrink;
develop bubble-like blebs on their surface;
have the chromatin (DNA and protein) in their nucleus degraded; have their mitochondria break down with the release of cytochrome c;
break into small, membrane-wrapped, fragments;
release (at least in mammalian cells) ATP and UTP.
These nucleotides bind to receptors on wandering phagocytic cells like macrophages and
dendritic cells and attract them to the dying cells (a " find-me " signal").
The phospholipid phosphatidylserine , which is normally hidden within the plasma membrane, is exposed on the surface.
This " eat me " signal is bound by other receptors on the phagocytes which then engulf the cell fragments.
The phagocytic cells secrete cytokines that inhibit inflammation (e.g., IL-10 and TGF-β)
7.
Explain measurement of cell growth
The cell growth can be detected by a variety of methods. The cell size growth can be visualized by Microscopy, using suitable stains. But the increase of cells number is usually more significative. It can be measured by manual counting of cells under microscopy observation, using the dye exclusion method (i.e. Trypan blue) to count only viable cells. Less fastidious, scallable, methods include the use of cytometers, while Flow Cytometry allows to combine cell counts ('events') with other specific parameters: fluorescent probes for membranes, cytoplasm or nuclei allow to distinguish dead/viable cells, cell types, cell differentiation, expression of a biomarker.
Beside the increasing number of cells, one can be assessed regarding the metabolic activity growth . I.e. the CFDA and Calcein-AM mesure (fluorimetrically) not only the membrane functionality (dye retention), but also the functionality of cytoplasmic enzymes (esterases). The
MTT assays (colorimetric) and the Resazurin assay (fluorimetric) dose the mitochondrial redox potentiel.
Finally, all these assays may correlate well, or not depending on cell growth conditions and desired aspects (activity, proliferation). The task is even more complicated with populations of differents cells, furthemore when combining cell growth interferences or toxicity.
8.
Write a note on enzymes used in disaggregation
• Enzymes
– Trypsin
– Collagenase II (from Pseudomonas perfringens)
– Elastase
– Hyaluronidase
– DNase
– Pronase (bacterial protease)
• Usually a combination of enzymes
• Crude preparations are usually more efficient
– The purer the less toxic
– The cruder the more effective due to contamination with other proteases
9.
Explain the collection of cells / tissue from chick embryo & define Secondary cell culture
Secondary cell culture is the culture derived from primary cell culture i.e., after first sub culturing.
The cells in a primary culture multiply and form a confluent layer. When subculturing is done from the primary cell culture it is called secondary cell culture.
When primary cells are again passaged in vitro , it is called secondary cell culture.
10.
Selection of Cell lines
General parameters for the selection of cell lines:
Finite vs infinite
Normal or transformed
Species
Growth characteristics
Availability
Validation
Phenotypic expression
Control cell lines
Stability
11.
Stem cell
Stem cells are biological cells found in all multicellular organisms, that can divide through mitosis and differentiate into diverse specialized cell types and can self renew to produce more stem cells.
The classical definition of a stem cell requires that it possess two properties:
Self-renewal - the ability to go through numerous cycles of cell division while maintaining the undifferentiated state.
Potency - the capacity to differentiate into specialized cell types. In the strictest sense, this requires stem cells to be either totipotent or pluripotent - to be able to give rise to any mature cell type, although multipotent or unipotent progenitor cells are sometimes referred to as stem cells.
Self-renewal
Two mechanisms exist to ensure that the stem cell population is maintained:
1.
Obligatory asymmetric replication - a stem cell divides into one father cell that is identical to the original stem cell, and another daughter cell that is differentiated
2.
Stochastic differentiation - when one stem cell develops into two differentiated daughter cells, another stem cell undergoes mitosis and produces two stem cells identical to the original.
Potency definitions
Pluripotent, embryonic stem cells originate as inner mass cells within a blastocyst. The stem cells can become any tissue in the body, excluding a placenta. Only the morula's cells are totipotent, able to become all tissues and a placenta.
Human embryonic stem cells
A: Cell colonies that are not yet differentiated. B: Nerve cell
Potency specifies the differentiation potential (the potential to differentiate into different cell types) of the stem cell.
Totipotent (a.k.a omnipotent) stem cells can differentiate into embryonic and extraembryonic cell types. Such cells can construct a complete, viable organism. These cells are produced from the fusion of an egg and sperm cell. Cells produced by the first few divisions of the fertilized egg are also totipotent.
Pluripotent stem cells are the descendants of totipotent cells and can differentiate into nearly all cells, i.e. cells derived from any of the three germ layers .
Multipotent stem cells can differentiate into a number of cells, but only those of a closely related family of cells.
Oligopotent stem cells can differentiate into only a few cells, such as lymphoid or myeloid stem cells.
Unipotent cells can produce only one cell type, their own, but have the property of selfrenewal which distinguishes them from non-stem cells (e.g. muscle stem cells).
12.
Cell viability assays
Assessed by dye inclusion and dye exclusion method.
Trypan blue exclusion assay
Trypan blue is a vital stain used to selectively colour dead tissues or cells blue. It is a diazo dye.
Live cells or tissues with intact cell membranes are not coloured. Since cells are very selective in the compounds that pass through the membrane, in a viable cell trypan blue is not absorbed; however, it traverses the membrane in a dead cell. Hence, dead cells are shown as a distinctive blue colour under a microscope. Since live cells are excluded from staining, this staining method is also described as a dye exclusion method.
propidium iodide (PI) exclusion assay
fluorescein diacetate (FDA) inclusion assay
13.
Cyto-toxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are a chemical substance, an immune cell or some types of venom (e.g. from the puff adder or brown recluse spider).
Measuring cytotoxicity
Cytotoxicity assays are widely used by the pharmaceutical industry to screen for cytotoxicity in compound libraries. Researchers can either look for cytotoxic compounds, if they are interested in developing a therapeutic that targets rapidly dividing cancer cells, for instance; or they can screen "hits" from initial high-throughput drug screens for unwanted cytotoxic effects before investing in their development as a pharmaceutical.
Assessing cell membrane integrity is one of the most common ways to measure cell viability and cytotoxic effects. Compounds that have cytotoxic effects often compromise cell membrane integrity. Vital dyes, such as trypan blue or propidium iodide are normally excluded from the inside of healthy cells; however, if the cell membrane has been compromised, they freely cross the membrane and stain intracellular components. Alternatively, membrane integrity can be assessed by monitoring the passage of substances that are normally sequestered inside cells to the outside. One commonly measured molecule is lactate dehydrogenase (LDH). Protease biomarkers have been identified that allow researchers to measure relative numbers of live and dead cells within the same cell population. The live-cell protease is only active in cells that have a healthy cell membrane, and loses activity once the cell is compromised and the protease is exposed to the external environment. The dead-cell protease cannot cross the cell membrane, and can only be measured in culture media after cells have lost their membrane integrity.
Cytotoxicity can also be monitored using the MTT or MTS assay. This assay measures the reducing potential of the cell using a colorimetric reaction. Viable cells will reduce the MTS reagent to a colored formazan product. A similar redox-based assay has also been developed using the fluorescent dye, resazurin. In addition to using dyes to indicate the redox potential of cells in order to monitor their viability, researchers have developed assays that use ATP content as a marker of viability. Such ATP-based assays include bioluminescent assays in which ATP is the limiting reagent for the luciferase reaction.
Cytotoxicity can also be measured by the sulforhodamine B (SRB) assay, WST assay and clonogenic assay.
A label-free approach to follow the cytotoxic response of adherent animal cells in real-time is based on electric impedance measurements when the cells are grown on gold-film electrodes.
This technology is referred to as electric cell-substrate impedance sensing (ECIS). Label-free real-time techniques provide the kinetics of the cytotoxic response rather than just a snapshot like many colorimetric endpoint assays.
Part – C
1.
Explain Biology of cultured cells
CELL ADHESION
CAM
cytoskeleton
CELL PROLIFERATION
Cell cycle
DIFFERENTIATION
Maintenance of differentiation
Dedifferentiation
2.
Write a note on Fundamentals, facilities and applications animal cell culture.
Mass culture of animal cell lines is fundamental to the manufacture of viral vaccines and many products of biotechnology. Biological products produced by recombinant DNA (rDNA) technology in animal cell cultures include enzymes, synthetic hormones, immunobiologicals
(monoclonal antibodies, interleukins, lymphokines), and anticancer agents. Although many simpler proteins can be produced using rDNA in bacterial cultures, more complex proteins that are glycosylated (carbohydrate-modified), currently must be made in animal cells. An important example of such a complex protein is the hormone erythropoietin. The cost of growing mammalian cell cultures is high, so research is underway to produce such complex proteins in insect cells or in higher plants.
Tissue culture and engineering
Cell culture is a fundamental component of tissue culture and tissue engineering, as it establishes the basics of growing and maintaining cells ex vivo .
Animal cell culture has become one of the major tools used in cell and molecular biology. Some of the important areas where:
Model system
Toxicity study
Cancer research
Virology
Cell-based manufacturing
Genetic counseling
Gene therapy
Drug screening and development
3.
Write a note on role of serum in animal cell culture media
It provides the basic nutrients for cells; the nutrients are present both in the solution as well as are bound to the proteins.
It provides several hormones, e.g., insulin, which is essential for growth of nearly all cells in culture, cortisone, testosterone, prostaglandin, etc.
It contains several growth factors, e.g., platelet derived growth factor (PDGF), transforming growth factor β (TGF- β), epidermal growth factor, etc.; these are present in concentrations of µg/l. Both hormones and growth factors are involved in growth promotion and specialized cell function. A given hormone or growth factor may stimulate growth of one cell type, may have no effect on another and may even be inhibitory to some others. For example, PDGF induces proliferation in fibroblasts, but induces differentiation of some types of epithelia.
Further, proliferation of a single cell type may be induced by more than one growth factor, e.g., fibroblasts respond to PDGF, epidermal growth factor, fibroblast growth factor and somatomidins
A major role of serum is to supply proteins, e.g., fibronectin, which promote attachment of cells to the substrate. It also provides spreading factors that help the cells to spread out before they can begin to divide. Although cells do produce these factors, but trypsinized cells are usually unable to attach to the substrate.
It provides several binding proteins, e.g., albumin, transferrin, etc., which carry other molecules into the cell. For example, albumin carries into cells lipids, vitamins, hormones, etc. Transferrin usually carries Fe in a nonbasic form, but binding of transferrin to its receptor in cell membrane is believed to be mitogenic.
It increases the viscosity of medium and, thereby, protects cells from mechanical damages, e.g., shear forces during agitation of suspension cultures.
Protease inhibitors present in the serum protect cells, especially trypsinised cells, from proteolysis.
The serum also provides minerals, like Na+, K+, Fe2+, Zn2+, etc.
It also acts as a buffer.
4.
Explain cell synchronization
Cell Synchronization is a process by which cells at different stages of the cell cycle in a culture are brought to the same phase. "Cell synchrony" is required to study the progression of cells through the cell cycle. The types of synchronizations are broadly categorized into two groups:
"Physical Fractionation" and "Chemical Blockade." Cell separation by physical means
Physical fractionation or cell separation techniques, based on the following characteristics are in use.
Cell density
Cell size
Affinity of antibodies on cell surface epitopes.
Light scatter or fluorescent emission by labeled cells.
The two commonly used techniques are:
Centrifugal separation
The physical characteristics - cell size and sedimentation velocity - are operative in the technique of centrifugal elutriation. Centrifugal elutriator (from Beckman) is an advanced device for increasing the sedimentation rate so that the yield and resolution of cells is better. The cell separation is carried out in a specially designed centrifuge and rotor.
Fluorescence-activated cell sorting
Fluorescence-activated cell sorting (FACS) is a technique for sorting out the cells based on the differences that can be detected by light scatter (eg. cell size) or fluorescence emission (by penetrated DNA, RNA, proteins, antigens). The procedure involves passing of a single stream of cells through a laser beam so that the scattered light from the cells can be detected and recorded.
There are two instruments in use based on its principle: a) Flow cytometer b) Fluorescence-activated cell sorter
Cell separation by chemical blockade
The cells can be separated by blocking metabolic reactions. Two types of metabolic blockades are in use:
Inhibition of DNA synthesis
During the S phase of cell cycle, DNA synthesis can be inhibited by using inhibitors such as thymidine, aminopterin, hydroxyurea and cytosine arabinoside. The effects of these inhibitors are variable. The cell cycle is predominantly blocked in S phase that results in viable cells.
Nutritional deprivation
Elimination of serum from the culture medium for about 24 hours results in the accumulation of cells at G1 phase. This effect of nutritional deprivation can be restored by their addition by which time the cell synchrony occurs.
5.
Explain the event of Apoptosis with diagram
Apoptosis is the process of programmed cell death (PCD) that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death.
These changes include blebbing, loss of cell membrane asymmetry and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation. (See also
Apoptosis DNA fragmentation.) Unlike necrosis, apoptosis produces cell fragments called apoptotic bodies that surrounding cells are able to engulf and quickly remove before the contents of the cell can spill out onto surrounding cells and cause damage.
Process
The process of apoptosis is controlled by a diverse range of cell signals, which may originate either extracellularly ( extrinsic inducers ) or intracellularly ( intrinsic inducers ). Extracellular signals may include toxins, hormones, growth factors, nitric oxide or cytokines, that must either cross the plasma membrane or transduce to effect a response. These signals may positively (i.e., trigger) or negatively (i.e., repress, inhibit, or dampen) affect apoptosis. (Binding and subsequent trigger of apoptosis by a molecule is termed positive induction , whereas the active repression or inhibition of apoptosis by a molecule is termed negative inductio n.)
A cell initiates intracellular apoptotic signalling in response to a stress, which may bring about cell suicide. The binding of nuclear receptors by glucocorticoids, heat, radiation, nutrient deprivation, viral infection, hypoxia and increased intracellular calcium concentration, for example, by damage to the membrane, can all trigger the release of intracellular apoptotic signals by a damaged cell. A number of cellular components, such as poly ADP ribose polymerase, may also help regulate apoptosis.
Before the actual process of cell death is precipitated by enzymes, apoptotic signals must cause regulatory proteins to initiate the apoptosis pathway. This step allows apoptotic signals to cause
cell death, or the process to be stopped, should the cell no longer need to die. Several proteins are involved, but two main methods of regulation have been identified: targeting mitochondria functionality, or directly transducing the signal via adaptor proteins to the apoptotic mechanisms.
Another extrinsic pathway for initiation identified in several toxin studies is an increase in calcium concentration within a cell caused by drug activity, which also can cause apoptosis via a calcium binding protease calpain.
6.
Organ culture
Organ culture is a development from tissue culture methods of research, the organ culture is able to accurately model functions of an organ in various states and conditions by the use of the actual in vitro organ itself.
Parts of an organ or a whole organ can be cultured in vitro . The main objective is to maintain the architecture of the tissue and direct it towards normal development. In this technique, it is essential that the tissue is never be disrupted or damaged. It thus requires careful handling. The media used for a growing organ culture are generally the same as those used for tissue culture.
The techniques for organ culture can be classified into (i) those employing a solid medium and
(ii) those employing liquid medium.
Embryonic organ culture is an easier alternative to normal organ culture derived from adult animals. The following are three techniques employed for embryonic organ culture.
Plasma clot method
The following are general steps in organ culture on plasma clots.
1.
Prepare a plasma clot by mixing 15 drops of plasma with five drops of embryo extract in a watch glass.
2.
Place a watch glass on a pad of cotton wool in a petri dish; cotton wool is kept moist to prevent excessive evaporation from the dish.
3.
Place a small, carefully dissected piece of tissue on top of the plasma clots in watch glass.
The technique has now been modified, and a raft of lens paper or rayon net is used on
which the tissue is placed. Transfer of the tissue can then be achieved by raft easily. Excessive fluid is removed and the net with the tissue placed again on the fresh pool of medium.Parts of an organ or a whole organ can be cultured in vitro. The main objective is to maintain the architecture of the tissue and direct it towards normal development. In this technique, it is essential that the tissue never be disrupted or damaged. It thus requires careful handling. The media used for a growing organ culture are generally the same as those used for tissue culture.
The techniques for organ culture can be classified into (i) those employing a solid medium and
(ii) those employing liquid medium.
Agar gel method
Media solidified with agar are also used for organ culture and these media consist of 7 parts 1% agar in BSS, 3 parts chick embryo extract and 3 parts of horse serum. Defined media with or without serum are also used with agar. The medium with agar provides the mechanical support for organ culture. It does not liquefy. Embryonic organs generally grow well on agar, but adult organ culture will not survive on this medium.
The culture of adult organs or parts from adult animals is more difficult due to their greater requirement of oxygen . A variety of adult organs (e.g. the liver ) have been cultured using special media with special apparatus (Towell’s II culture chamber).
Since serum was found to be toxic, serum-free media were used, and the special apparatus permitted the use of 95% oxygen.
Raft Methods
In this approach the explant is placed onto a raft of lens paper or rayon acetate, which is floated on serum in a watch glass. Rayon acetate rafts are made to float on the serum by treating their 4 corners with silicone.
Similarly, floatability of lens paper is enhanced by treating it with silicone. On each raft, 4 or more explants are usually placed.
In a combination of raft and clot techniques, the explants are first placed on a suitable raft, which is then kept on a plasma clot. This modification makes media changes easy, and prevents the sinking of explants into liquefied plasma.
Grid Method mm
Initially devised by Trowell in
1954, the grid method utilizes 25 x 25 mm pieces of a suitable wire mesh or perforated stainless steel sheet whose edges are bent to form 4 legs of about 4 mm height.
Skeletal tissues are generally placed directly on the grid but softer tissues like glands or skin are first placed on rafts, which are then kept on the grids.
The grids themselves are placed in a culture chamber filled with fluid medium up to the grid; the chamber is supplied with a mixture of O
2
and CO
2
to meet the high O
2
requirements of adult mammalian organs. A modification of the original grid method is widely used to study the growth and differentiation of adult and embryonic tissues.
Limitations
Results from organ cultures are often not comparable to those from whole animals studies, e.g. in studies on drug action since the drugs are metabolized in vivo but not in vitro.
7.
What are the steps involved in Thawing
After cryopreservation thawing (defrost) is done when we need the preserved cell lines for use.
Defrost the vial in a 37°C water bath with constant, moderate agitation, until ice in the ampule is no longer visible.
Thawing Cryopreserved Cells
1. Defrost the vial in a 37°C water bath with constant, moderate agitation, until ice in the ampule is no longer visible.
2. Continue to warm the ampule in the water bath for 30 seconds with gentle agitation.
3. Immediately disinfect the vial with 70% ethanol.
4. Working in a hood, open the vial and transfer the contents to a sterile 15 ml tube. (Note: if thawing a glass vial, open the vial by wrapping the disinfected vial in a sterile gauze pad and breaking the neck of the pre-scored ampule).
5. Add 1.5 ml of culture medium (serum containing medium) that has been pre-warmed to
37°C.
6. Allow to stand for 5 minutes.
7. Add 3 ml of prewarmed culture medium and allow to stand for 5 minutes.
8. Add 6 ml of prewarmed culture medium and allow to stand for 5 minutes.
9. Centrifuge the suspended cells at 200 x g for 10 minutes.
10. Decant the medium and gently resuspend the cell pellet in 25 ml of culture medium and transfer into one 75 cm^2 culture flask.
11. Observe the cells microscopically to estimate cell viability and then place them in an incubator.
12. After overnight incubation, the cells should be observed so that the viability may again be evaluated. A trypan blue dye exclusion stain may be appropriate when precise viability assay is desired.
14.
Types of cell culture
Based on the origin of the cells
Primary culture (directly from animal or plant tissue)
Extended culture (multipassage culture) – cell strain
Established (transformed) cell lines
Primary culture (directly from animal or plant tissue):
A culture derived directly from a tissue, cells organ from an organism and before first sub culturing.
A stage from cell isolation to first subculturing
Carrot callus growth was primary plant culture
When cultures are established initially from tissue taken directly from animals, they contain several cell types, most of which are capable of only 5-10 divisions. Due to high cost, inconvenience of getting fresh tissue each time and variation from batch to batch, it is not suitable for use in routine diagnostic work or vaccine production. Furthermore, the donor animals may harbour the latent viruses which can confuse diagnosis or contaminate vaccines.
But the primary cultures are very sensitive to many human and veterinary viruses and still used for primary isolation of these viruses. Secondary cell culture : When primary cells are again passaged in vitro , it is called secondary cell culture. Diploid cell strains: These are cells that are capable of undergoing a number of divisions in culture that is routinely related to the life span of the species of animals- about 50 for fetal human cells and about 10 for fetal cells from horses and cows. Diploid cell strains of fibroblasts established from human fetuses or embryos are widely used in human diagnostic virology and vaccine production, but diploid strains have not been much used in veterinary vaccine production.
Extended culture (multipassage culture) – cell strain
Established (transformed or Continuous) cell line: These are cells of a single type that are capable of indefinite propagation in vitro. Such immortal cell lines originate from cancers or by spontaneous transformation of a diploid cell strain. They do not bear the close resemblance to
their cell of origin as they undergo many mutations during their prolonged culture. The cells usually have lost the specialized morphology and biochemical abilities. Cells of continuous cell lines are often aneuploid in chromosome number, especially if of malignant origin. Continuous cell lines derived from monkey (MA 104, Vero), dog (MDCK), cattle (MDBK), pig (PK15), Cat
(CRFK), mouse (L929, 3T3, hamster (BHK21), rabbit (RK-13), and others are widely used in experimental and diagnostic virology. The great advantage of continuous cell lines over primary cell cultures is that they can be propagated indefinitely by subculturing the cells at regular intervals. Like other cells, they can be preserved for many years when frozen in serum containing medium with added glycerol or DMSO and stored at –80°C or –196°C (liquid nitrogen).
15.
Primary cell culture
•
A culture derived directly from a tissue, cells organ from an organism and before first sub culturing.
A stage from cell isolation to first subculturing
Carrot callus growth was primary plant culture
• Best resembling natural tissue
• Limited growth potential
• Limited life span
• May give rise to a cell strain or be immortalized
• Strain – a lineage of cells originating from one primary culture.
Unit IV
Transgenic animals: Method of obtaining transgenic animals using fertilized eggs and embryonic blastocyst cell, example, importance of transgenic animals – increased productivity of domestic animals, improved desired characters of domestic animals, production of recombinant gene products and proteins for pharmaceutical use. Animal models for tackling human diseases
(Gene knock out in mice models).
Part – A
1.
Transgenic animals
The term transgenic animal refers to an animal in which there has been a deliberate modification of the genome - the material responsible for inherited characteristics - in contrast to spontaneous mutation
2.
Cytokines
They are small cell -signaling protein molecules that are secreted by the glial cells of the nervous system and by numerous cells of the immune system and are a category of signaling molecules used extensively in intercellular communication. Cytokines can be classified as proteins , peptides , or glycoproteins ; the term "cytokine" encompasses a large and diverse family of regulators produced throughout the body by cells of diverse embryological origin. The term "cytokine" has been used to refer to the immunomodulating agents, such as interleukins and interferons .
3.
Plasminogen activators
A plasminogen activator is a serine protease which converts plasminogen to plasmin, thus promoting fibrinolysis . Plasminogen activators form a key component of fibrinolytic system with a high specificity for plasminogen yielding the active enzyme plasmin through the hydrolysis of the Arg560 - Val561 peptide bond.
4.
Types of Plasminogen activators
Tissue plasminogen activator
Urokinase
It is inhibited by plasminogen activator inhibitor-1 and plasminogen activator inhibitor-2 .
5.
Tissue plasminogen activator
Tissue plasminogen activator (abbreviated TPA or PLAT) is a protein involved in the breakdown of blood clots . It is a serine protease ( EC 3.4.21.68
) found on endothelial cells , the cells that line the blood vessels . As an enzyme , it catalyzes the conversion of plasminogen to plasmin , the major enzyme responsible for clot breakdown. Because it works on the clotting system , tPA is used in clinical medicine to treat only embolic or thrombotic stroke . Use is contraindicated in hemorrhagic stroke and head trauma. tPA may be manufactured using recombinant biotechnology techniques. tPA created this way may be referred to as recombinant tissue plasminogen activator ( rtPA ).
The classic role of tPA is in the clotting system . To be specific, tPA catalyzes the conversion of plasminogen into plasmin . It does so by cleaving the single-chain plasminogen into two chains.
These two chains are linked by a disulfide bond and the resulting molecule is called plasmin.
Increased enzymatic activity causes hyperfibrinolysis , which manifests as excessive bleeding.
Decreased activity leads to hypofibrinolysis which can result in thrombosis or embolism .
Tissue plasminogen activator also plays a role in cell migration and tissue remodeling . tPA is used in diseases that feature blood clots , such as pulmonary embolism , myocardial infarction , and stroke , in a medical treatment called thrombolysis .
Recombinant tissue plasminogen activators
Recombinant tissue plasminogen activators (r-tPAs) include alteplase , reteplase , and tenecteplase
(TNKase). Alteplase is FDA-approved for treatment of myocardial infarction with ST-elevation
(STEMI), acute ischemic stroke (AIS), acute massive pulmonary embolism , and central venous access devices (CVAD).
Reteplase is FDA-approved for acute myocardial infarction , where it has more convenient administration and faster thrombolysis than alteplase. Tenecteplase is also indicated in acute myocardial infarction , showing fewer bleeding complications but otherwise similar mortality rates after one year compared to alteplase.
Additional r-tPAs, such as desmoteplase , are under clinical development.
6.
Urokinase
It also called urokinase-type plasminogen activator (uPA), is a serine protease ( EC 3.4.21.73
). Urokinase was originally isolated from human urine , but is present in several physiological locations, such as blood stream and the extracellular matrix . The primary physiological substrate is plasminogen , which is an inactive zymogen form of the serine protease plasmin . Activation of plasmin triggers a proteolysis cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This links urokinase to vascular diseases and cancer.
Urokinase is used clinically as a thrombolytic agent in the treatment of severe or massive deep venous thrombosis , pulmonary embolism , myocardial infarction , and occluded intravenous or dialysis cannulas.
It is also administered intrapleurally to improve the drainage of complicated pleural effusions and empyemas.
7.
Coagulation
Coagulation is a complex process by which blood forms clots . It is an important part of hemostasis (the cessation of blood loss from a damaged vessel), wherein a damaged blood vessel wall is covered by a platelet and fibrin -containing clot to stop bleeding and begin repair of the damaged vessel. Disorders of coagulation can lead to an increased risk of bleeding ( hemorrhage ) or obstructive clotting ( thrombosis ).
8.
Factor VIII
Factor VIII turned out to be deficient in the clinically recognised but etiologically elusive hemophilia A ; it was identified in the 1950s and is alternatively called antihemophilic globulin due to its capability to correct hemophilia A
9.
Factor IX
Factor IX was discovered in 1952 in a young patient with hemophilia B named Stephen
Christmas (1947-1993). His deficiency was described by Dr. Rosemary Biggs and Professor
R.G. MacFarlane in Oxford, UK. The factor is, hence, called Christmas Factor. Christmas lived in Canada, and campaigned for blood transfusion safety until succumbing to transfusion-related
AIDS at age 46. An alternative name for the factor is plasma thromboplastin component , given by an independent group in California.
10.
Growth harmones
A hormone (from Greek ὁρμή
"impetus") is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism . In essence, it is a chemical messenger that transports a signal from one cell to another. All multicellular organisms produce hormones; plant hormones are also called phytohormones . Hormones in animals are often transported in the blood. Cells respond to a hormone when they express a specific receptor for that hormone. The hormone binds to the receptor protein , resulting in the activation of a signal transduction mechanism that ultimately leads to cell type-specific responses.
Endocrine hormone molecules are secreted (released) directly into the bloodstream , whereas exocrine hormones (or ectohormones) are secreted directly into a duct, and, from the duct, they flow either into the bloodstream or from cell to cell by diffusion in a process known as paracrine signalling .
Recently it has been found that a variety of exogenous modern chemical compounds have hormone-like effects on both humans and wildlife. Their interference with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body can change the homeostasis, reproduction, development, and/or behavior the same as endogenous produced hormones."
Part – B
1.
Transgenic animals
The term transgenic animal refers to an animal in which there has been a deliberate modification of the genome - the material responsible for inherited characteristics - in contrast to spontaneous mutation (FELASA September 1992, revised February 1995). Foreign DNA is introduced into the animal, using recombinant DNA technology, and then must be transmitted through the germ line so that every cell, including germ cells, of the animal contain the same modified genetic material.
Prior to the development of molecular genetics, the only way of studying the regulation and function of mammalian genes was through the observation of inherited characteristics or spontaneous mutations. Long before Mendel and any molecular genetic knowledge, selective breeding was a common practice among farmers for the enhancement of chosen traits, e.g., increased milk production.
During the 1970s, the first chimeric mice were produced (Brinster, 1974). The cells of two different embryos of different strains were combined together at an early stage of development
(eight cells) to form a single embryo that subsequently developed into a chimeric adult, exhibiting characteristics of each strain.
The mutual contributions of developmental biology and genetic engineering permitted rapid development of the techniques for the creation of transgenic animals. DNA microinjection, the first technique to prove successful in mammals, was first applied to mice (Gordon and Ruddle,
1981) and then to various other species such as rats, rabbits, sheep, pigs, birds, and fish. Two other main techniques were then developed: those of retrovirus-mediated transgenesis (Jaenisch,
1976) and embryonic stem (ES) cell-mediated gene transfer (Gossler et al., 1986).
Since 1981, when the term transgenic was first used by J.W. Gordon and F.H. Ruddle (1981), there has been rapid development in the use of genetically engineered animals as investigators have found an increasing number of applications for the technology.
Methods of creation of transgenic animals
For practical reasons, i.e., their small size and low cost of housing in comparison to that for larger vertebrates, their short generation time, and their fairly well defined genetics, mice have become the main species used in the field of transgenics.
The three principal methods used for the creation of transgenic animals are DNA microinjection, embryonic stem cell-mediated gene transfer and retrovirus-mediated gene transfer. a) DNA microinjection. b) Embryonic stem cell-mediated gene transfer. c) Retrovirus-mediated gene transfer.
To increase the probability of expression, gene transfer is mediated by means of a carrier or vector, generally a virus or a plasmid. Retroviruses are commonly used as vectors to transfer genetic material into the cell, taking advantage of their ability to infect host cells in this way.
Offspring derived from this method are chimeric, i.e., not all cells carry the retrovirus.
Transmission of the transgene is possible only if the retrovirus integrates into some of the germ cells.
For any of these techniques the success rate in terms of live birth of animals containing the transgene is extremely low. Providing that the genetic manipulation does not lead to abortion, the result is a first generation (F1) of animals that need to be tested for the expression of the transgene. Depending on the technique used, the F1 generation may result in chimeras. When the transgene has integrated into the germ cells, the so-called germ line chimeras are then inbred for
10 to 20 generations until homozygous transgenic animals are obtained and the transgene is present in every cell. At this stage embryos carrying the transgene can be frozen and stored for subsequent implantation.
2.
Plasminogen activators & its types
A plasminogen activator is a serine protease which converts plasminogen to plasmin, thus promoting fibrinolysis . Plasminogen activators form a key component of fibrinolytic system with a high specificity for plasminogen yielding the active enzyme plasmin through the hydrolysis of the Arg560 - Val561 peptide bond.
Types of Plasminogen activators
Tissue plasminogen activator
Urokinase
It is inhibited by plasminogen activator inhibitor-1 and plasminogen activator inhibitor-2 .
3.
Tissue plasminogen activator
Tissue plasminogen activator (abbreviated TPA or PLAT) is a protein involved in the breakdown of blood clots . It is a serine protease ( EC 3.4.21.68
) found on endothelial cells , the cells that line the blood vessels . As an enzyme , it catalyzes the conversion of plasminogen to plasmin , the major enzyme responsible for clot breakdown. Because it works on the clotting system , tPA is used in clinical medicine to treat only embolic or thrombotic stroke . Use is contraindicated in hemorrhagic stroke and head trauma. tPA may be manufactured using recombinant biotechnology techniques. tPA created this way may be referred to as recombinant tissue plasminogen activator ( rtPA ).
The classic role of tPA is in the clotting system . To be specific, tPA catalyzes the conversion of plasminogen into plasmin . It does so by cleaving the single-chain plasminogen into two chains.
These two chains are linked by a disulfide bond and the resulting molecule is called plasmin.
Increased enzymatic activity causes hyperfibrinolysis , which manifests as excessive bleeding.
Decreased activity leads to hypofibrinolysis which can result in thrombosis or embolism .
Tissue plasminogen activator also plays a role in cell migration and tissue remodeling . tPA is used in diseases that feature blood clots , such as pulmonary embolism , myocardial infarction , and stroke , in a medical treatment called thrombolysis .
4. Recombinant tissue plasminogen activators
Recombinant tissue plasminogen activators (r-tPAs) include alteplase , reteplase , and tenecteplase
(TNKase). Alteplase is FDA-approved for treatment of myocardial infarction with ST-elevation
(STEMI), acute ischemic stroke (AIS), acute massive pulmonary embolism , and central venous access devices (CVAD).
Reteplase is FDA-approved for acute myocardial infarction , where it has more convenient administration and faster thrombolysis than alteplase. Tenecteplase is also indicated in acute myocardial infarction , showing fewer bleeding complications but otherwise similar mortality rates after one year compared to alteplase.
Additional r-tPAs, such as desmoteplase , are under clinical development.
5.
Urokinase
It also called urokinase-type plasminogen activator (uPA), is a serine protease ( EC 3.4.21.73
). Urokinase was originally isolated from human urine , but is present in several physiological locations, such as blood stream and the extracellular matrix . The primary physiological substrate is plasminogen , which is an inactive zymogen form of the serine protease plasmin . Activation of plasmin triggers a proteolysis cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This links urokinase to vascular diseases and cancer.
Urokinase is used clinically as a thrombolytic agent in the treatment of severe or massive deep venous thrombosis , pulmonary embolism , myocardial infarction , and occluded intravenous or dialysis cannulas.
It is also administered intrapleurally to improve the drainage of complicated pleural effusions and empyemas
6.
Blood clotting factors
Blood clotting factors / Coagulation factors and related substances
Number and/or name
I ( fibrinogen )
Function
Forms clot (fibrin)
II (
Tissue factor
Calcium
V prothrombin )
(proaccelerin, labile factor)
Its active form (IIa) activates I , V , VII , VIII , XI , XIII , protein C , platelets
Co-factor of VIIa (formerly known as factor III )
Required for coagulation factors to bind to phospholipid
(formerly known as factor IV)
Co-factor of X with which it forms the prothrombinase complex
VI
VII (stable factor, proconvertin)
Unassigned – old name of Factor Va
Activates IX , X
VIII (Antihemophilic factor A) Co-factor of IX with which it forms the tenase complex
IX (Antihemophilic factor B or Christmas
Activates X: forms tenase complex with factor VIII factor)
X (Stuart-Prower factor) Activates II: forms prothrombinase complex with factor V
XI (plasma thromboplastin antecedent) Activates IX
XII (Hageman factor)
XIII (fibrin-stabilizing factor) von Willebrand factor prekallikrein (Fletcher factor)
Activates factor XI, VII and prekallikrein
Crosslinks fibrin
Binds to VIII, mediates platelet adhesion
Activates XII and prekallikrein; cleaves HMWK high-molecular-weight kininogen (HMWK)
Supports reciprocal activation of XII, XI, and prekallikrein
(Fitzgerald factor) fibronectin antithrombin III heparin cofactor II
Mediates cell adhesion
Inhibits IIa, Xa, and other proteases;
Inhibits IIa, cofactor for heparin and dermatan sulfate
("minor antithrombin") protein C protein S
Inactivates Va and VIIIa
Cofactor for activated protein C (APC, inactive when bound to C4b-binding protein)
protein Z
Mediates thrombin adhesion to phospholipids and stimulates degradation of factor X by ZPI
Protein Z-related protease inhibitor (ZPI)
Degrades factors X (in presence of protein Z) and XI
(independently) plasminogen alpha 2-antiplasmin tissue plasminogen activator (tPA)
Converts to plasmin, lyses fibrin and other proteins
Inhibits plasmin
Activates plasminogen urokinase Activates plasminogen plasminogen activator inhibitor-1 (PAI1) Inactivates tPA & urokinase (endothelial PAI) plasminogen activator inhibitor-2 (PAI2) Inactivates tPA & urokinase ( placental PAI) cancer procoagulant Pathological factor X activator linked to thrombosis in cancer
The remainder of the biochemical factors in the process of coagulation were largely discovered in the 20th century.
A first clue as to the actual complexity of the system of coagulation was the discovery of proaccelerin (initially and later called Factor V) by Paul Owren (1905-1990) in 1947. He also postulated its function to be the generation of accelerin (Factor VI), which later turned out to be the activated form of V (or Va); hence, VI is not now in active use.
Factor VII (also known as serum prothrombin conversion accelerator or proconvertin , precipitated by barium sulfate) was discovered in a young female patient in 1949 and 1951 by different groups.
Factor VIII turned out to be deficient in the clinically recognised but etiologically elusive hemophilia A ; it was identified in the 1950s and is alternatively called antihemophilic globulin due to its capability to correct hemophilia A.
Factor IX was discovered in 1952 in a young patient with hemophilia B named Stephen
Christmas (1947-1993). His deficiency was described by Dr. Rosemary Biggs and Professor
R.G. MacFarlane in Oxford, UK. The factor is, hence, called Christmas Factor. Christmas lived in Canada, and campaigned for blood transfusion safety until succumbing to transfusion-related
AIDS at age 46. An alternative name for the factor is plasma thromboplastin component , given by an independent group in California.
Hageman factor, now known as factor XII, was identified in 1955 in an asymptomatic patient with a prolonged bleeding time named of John Hageman. Factor X, or Stuart-Prower factor, followed, in 1956. This protein was identified in a Ms. Audrey Prower of London, who had a lifelong bleeding tendency. In 1957, an American group identified the same factor in a Mr. Rufus
Stuart. Factors XI and XIII were identified in 1953 and 1961, respectively.
The view that the coagulation process is a "cascade" or "waterfall" was enunciated almost simultaneously by MacFarlane in the UK and by Davie and Ratnoff in the USA, respectively.
7.
Role of Human Growth harmones
Human growth hormone plays several significant roles in:
Conversion of body fat to muscle mass
Energy level
Growth of all tissues
Tissue repair
Cell replacement
Enzyme production
Whole body healing
Bone strength
Sexual function
Brain function
Organ health and integrity
Integrity of nails, hair, skin and vital organs
1.
Methods to produce Transgenic animals
Part – C
The term transgenic animal refers to an animal in which there has been a deliberate modification of the genome - the material responsible for inherited characteristics - in contrast to spontaneous mutation.
Methods of creation of transgenic animals
For practical reasons, i.e., their small size and low cost of housing in comparison to that for larger vertebrates, their short generation time, and their fairly well defined genetics, mice have become the main species used in the field of transgenics.
The three principal methods used for the creation of transgenic animals are DNA microinjection, embryonic stem cell-mediated gene transfer and retrovirus-mediated gene transfer. a) DNA microinjection.
This method involves the direct microinjection of a chosen gene construct (a single gene or a combination of genes) from another member of the same species or from a different species, into the pronucleus of a fertilized ovum. It is one of the first methods that proved to be effective in mammals (Gordon and Ruddle, 1981). The introduced DNA may lead to the over- or underexpression of certain genes or to the expression of genes entirely new to the animal species. The insertion of DNA is, however, a random process, and there is a high probability that the introduced gene will not insert itself into a site on the host DNA that will permit its expression.
The manipulated fertilized ovum is transferred into the oviduct of a recipient female, or foster mother that has been induced to act as a recipient by mating with a vasectomized male.
A major advantage of this method is its applicability to a wide variety of species. b) Embryonic stem cell-mediated gene transfer.
This method involves prior insertion of the desired DNA sequence by homologous recombination into an in vitro culture of embryonic stem (ES) cells. Stem cells are
undifferentiated cells that have the potential to differentiate into any type of cell (somatic and germ cells) and therefore to give rise to a complete organism. These cells are then incorporated into an embryo at the blastocyst stage of development. The result is a chimeric animal. ES cellmediated gene transfer is the method of choice for gene inactivation, the so-called knock-out method.
This technique is of particular importance for the study of the genetic control of developmental processes. This technique works particularly well in mice. It has the advantage of allowing precise targeting of defined mutations in the gene via homologous recombination. c) Retrovirus-mediated gene transfer.
To increase the probability of expression, gene transfer is mediated by means of a carrier or vector, generally a virus or a plasmid. Retroviruses are commonly used as vectors to transfer genetic material into the cell, taking advantage of their ability to infect host cells in this way.
Offspring derived from this method are chimeric, i.e., not all cells carry the retrovirus.
Transmission of the transgene is possible only if the retrovirus integrates into some of the germ cells.
For any of these techniques the success rate in terms of live birth of animals containing the transgene is extremely low. Providing that the genetic manipulation does not lead to abortion, the result is a first generation (F1) of animals that need to be tested for the expression of the transgene. Depending on the technique used, the F1 generation may result in chimeras. When the transgene has integrated into the germ cells, the so-called germ line chimeras are then inbred for
10 to 20 generations until homozygous transgenic animals are obtained and the transgene is present in every cell. At this stage embryos carrying the transgene can be frozen and stored for subsequent implantation.
2.
Applications of Transgenic animals
Transgenic animals are just one in a series of developments in the area of biotechnology.
Biotechnology has transformed the way in which we understand processes such as engineering and manufacturing. These terms now include the use of living organisms or their parts to make or modify products, to change the characteristics of plants or animals, or to develop micro-
organisms for specific uses. The novel uses of biological techniques such as recombinant DNA techniques, cell fusion techniques, mono and polyclonal antibody technology and biological processes for commercial production have altered traditional distinctions and methods (US
Congress, Office of Technology Assessment, 1989). Genetic manipulations at the level of DNA have also changed long held views as to what is considered to be animal, plant and human. In turn, these changes have made it more difficult to evaluate the ways in which animals are used and have obscured distinctions between pure and applied research.
Consideration of the acceptability of creating specific transgenic animal strains or genetic manipulation involving interchanging DNA between species and kingdoms could be a simple animal care issue or a societal decision. The following is an attempt to show what the ability to create transgenic animals or engage in other forms of DNA manipulation means in terms of traditional ACC functions, not forgetting that this impacts on wider considerations of human responsibility for the welfare of other life forms.
The creation of transgenic animals is resulting in a shift from the use of higher order species to lower order species, and is also affecting the numbers of animals used. This shift in the patterns of animal use is being monitored by the CCAC through the use of the Animal Use Data Form.
An example of the replacement of higher species by lower species is the possibility to develop disease models in mice rather than using dogs or non-human primates.
In the long term, a reduction in the number of animals used, for example to study human diseases, is possible due to a greater specificity of the transgenic models developed. On the other hand, the success of the method has led to using its potential for investigating a wider range of diseases and conditions. The actual use of some species may be increased, in addition to the numbers of animals which are sacrificed as donors during the creation process. The potential of the technology has also made it possible to consider employing cattle, swine, sheep and goats as processing units to manufacture proteins or as organ donors.
The complex interactive processes of living mammals are not reproducible in vitro. However, transgenic animals provide a means of evaluating genetic modifications in terms of anatomical and physiological changes in a complex system. Transgenic models are more precise in
comparison to traditional animal models, for example the oncomouse with its increased susceptibility to tumor development enables results for carcinogenicity studies to be obtained within a shorter time-frame, thus reducing the course of tumor development in experimentally affected animals. However, models are not strict equivalents, so as with any other system care must be taken in drawing conclusions from the data.
A representative, but non-inclusive, list of purposes for which transgenic animals have been used indicates the wide ranging application of this biotechnology:
in medical research, transgenic animals are used to identify the functions of specific factors in complex homeostatic systems through over- or under-expression of a modified gene (the inserted transgene);
in toxicology: as responsive test animals (detection of toxicants);
in mammalian developmental genetics;
in molecular biology, the analysis of the regulation of gene expression makes use of the evaluation of a specific genetic change at the level of the whole animal;
in the pharmaceutical industry, targeted production of pharmaceutical proteins, drug production and product efficacy testing; in biotechnology: as producers of specific proteins;
genetically engineered hormones to increase milk yield, meat production; genetic engineering of livestock and in aquaculture affecting modification of animal physiology and/or anatomy; cloning procedures to reproduce specific blood lines; and developing animals specially created for use in xenografting.
Important general considerations include the extent to which experience acquired in the laboratory with regard to husbandry should influence industry standards for keeping animals created specifically as living machines for the production of proteins, antibodies, etc. What words are appropriate to describe and evaluate the condition of animals now used as production units? The successful cloning of Dolly underlines the fact that innovative developments in animal science are part of the mainstream of biotechnology. In addition, the use of xenografts, at least at the public health level makes animal and human welfare inseparable.
3.
Cytokines
They are small cell -signaling protein molecules that are secreted by the glial cells of the nervous system and by numerous cells of the immune system and are a category of signaling molecules used extensively in intercellular communication. Cytokines can be classified as proteins , peptides , or glycoproteins ; the term "cytokine" encompasses a large and diverse family of regulators produced throughout the body by cells of diverse embryological origin. The term "cytokine" has been used to refer to the immunomodulating agents, such as interleukins and interferons .
Structure: Structural homology has been able to partially distinguish between cytokines that do not demonstrate a considerable degree of redundancy so that they can be classified into four types:
The fourα-helix bundle family - Member cytokines have three-dimensional structures with four bundles of α-helices . This family, in turn, is divided into three sub-families:
1.
the IL-2 subfamily
2.
the interferon (IFN) subfamily
3.
the IL-10 subfamily. o The first of these three subfamilies is the largest. It contains several non-immunological cytokines including erythropoietin (EPO) and thrombopoietin (TPO). Also, four α-helix bundle cytokines can be grouped into long-chain and short-chain cytokines. the IL-1 family, which primarily includes IL-1 and IL-18 the IL-17 family, which has yet to be completely characterized, though member cytokines have a specific effect in promoting proliferation of T-cells that cause cytotoxic effects
Function:
A classification that proves more useful in clinical and experimental practice divides immunological cytokines into those that enhance cellular immune responses, type 1 (IFN-γ,
TGF-β, etc.), and type 2 ( IL-4 , IL-10 , IL-13 , etc.), which favor antibody responses.
A key focus of interest has been that cytokines in one of these two sub-sets tend to inhibit the effects of those in the other. Dysregulation of this tendency is under intensive study for its possible role in the pathogenesis of autoimmune disorders .
Several inflammatory cytokines are induced by oxidant stress . The fact that cytokines themselves trigger the release of other cytokines and also lead to increased oxidant stress makes them important in chronic inflammation .
4.
Growth harmones
Human growth hormone, also called as somatrophin is a protein, which contains 191 amino acids and is usually found in children and young adults at it's highest levels. A human body naturally produces it to help fuel growth during childhood period and to maintain organs and tissues of the body for whole life.
The pea-sized structure present at the base of your brain is known as pituitary gland that produces largest amount of hgh. This gland slowly reduces the amount of hormone when human reach at his 40s.
HGH is a very complex hormone, as it is made up of 191 amino acids. You can say that it is one of the largest hormone or the largest protein created by the pituitary gland.
Secretion of growth hormone reaches at the highest point during adolescence. This makes sense because this hormone assists stimulation of our body to grow, but the secretion of it does not stop after adolescence. Usually, our body produces it in short bursts during deep sleep.
Generally, any part or anything that goes in our body is related to growth hormone in some way. That's why human growth hormone often called as the fountain of youth as you feel younger with its elevated levels.
This hormone is produced in a person's body throughout his lifetime but during his youth it exists in a great quantity. It induces growth in children and plays a very significant role in adult metabolism.
Human growth hormone known to be critical for muscle growth, tissue repair, healing, bone strength, brain function, physical and mental strength, energy and metabolism. Actually, it is very important factor to just about every aspect of our life.
In 1996 scientists first isolated this hormone. It was started to use in the treatments for the children suffering from stunted growth problems. The only source to produce HGH was human corpses before the arrival of genetic engineering. The pituitary glands from the human body are removed from cadavers, then these glands are processed and the hormones are made to be available in injectable forms.
The growth hormone is mostly used for short children, but now days other people have found various uses of it. All types of sportsman and athletes were started to use it to boost their performance in various sports.
The main use of HGH is to increase muscle size. The competitors in many sports need power and strength would be mostly benefited because of the correlation between the strength and muscle size of a human body.
It also helps to recover the tired muscles quickly and allows you to train harder and more often. But in the endurance sports it is not used mostly instead of it EPO has bee favoured, which maximizes the oxygen carrying capacity and thus stamina.
Unit V
Transgenic silkworms. Animal cloning: Methods of cloning in animal system – Rat, Sheep, pig; importance of cloning. Gene therapy and cell mediated therapy, RFLP maps, RAPD markers, PCR, antisense technology, terminator gene technology, DNA finger printing.
Part A
1.
Stem cells: Stem cells in the human body have a unique ability to renew themselves and give rise to the more specialized cell types that do the work of the body. Stem cells remain unspecialized until a signal from the body tells them to develop into specific cells of the body like a heart, nerve, or skin cell.
2.
What is cloning?
Reproductive cloning- The entire animal is produced from a single cell by asexual reproduction. This would allow for the creation of a human being who is genetically identical to another.
Therapeutic cloning- Broader use of the term “cloning.” Does not create a new genetically identical individual. Research includes therapy for human mitochondria disease and others that could replace damaged or diseased tissues without the risk of rejecting another’s tissue. Could create new skin tissue for burn patients.
3.
Part B
1.
Methods of cloning
Embryo splitting- Artificially splitting a single embryo at a very early stage of development. In the natural process this would create twins. However, because this is done at an early stage and there are usually less than eight cells you can only make a few clones. Both the nuclear genes and mitochondria genes would be identical.
2.
Types of Stem Cells
Derivation
Method hES Cells
Removal of cells from ICM of blastocyst embryo from IVF.
SCNT ES Cells
Somatic Cell Nuclear
Transfer. Transfer of somatic cell nucleus to enucleated egg, development to blastocyst, removal of
ICM. iPS Cells
Reprogramming of somatic cells by introduction of specific regulatory factor genes.
Adult Stem Cells
Isolation from adult tissues.
Characteristics Differentiate into all cell types.
Excess of IVF embryos exist.
Differentiate into all cell types.
Stem cells can be matched to patient
Limitations Limited number of lines available for federally funded research.
Immune rejection issues
Risk of tumors
(teratomas) from transplanting undifferentiated cells.
Requires use of embryo.
No human SCNT cell lines exist.
Risk of
(teratomas) tumors from transplanting undifferentiated cells.
Eggs difficult to obtain.
Part C
Somatic Cell Nuclear Transfer Embryonic Stem Cells (SCNT SE Cells)
“ES cell – like” characteristics.
Stem cells can be matched to patient
Doesn't embryos. require
Unknown if cells can differentiate into all cell types.
Risk of tumors
(teratomas) from transplanting undifferentiated cells and from expression of introduced genes.
Successful treatments demonstrated.
Stem cells can be matched to patient
Cells not found in all tissues.
Produce a limited number of cell types.
Difficult to identify, isolate and grow.
Somatic cell nuclear transfer (SCNT) is a laboratory procedure that produces a blastocyst from an unfertilized egg and an ordinary adult somatic cell (e.g., from a single skin cell)
While SCNT techniques have been utilized in animal research for many years, human pluripotent stem cells were only first derived from SCNT-blastocysts in 2004 by a South
Korean research team.
SCNT substitutes the nucleus of a somatic cell (which contains all the genetic information of the patient) for the nucleus of a donated egg that has not been fertilized. In cell culture, this customized egg is then coaxed with an electronic or chemical catalyst to develop into a zygote as if it had been fertilized. The zygote begins cell division and develops into a ball of cells called the morula and then into the blastocyst at approximately five days. The inner cell mass of the blastocyst is then removed to generate a pluripotent stem cell line. After the inner cell mass is removed, the blastocyst is no longer capable of further development.