Science Class
advertisement

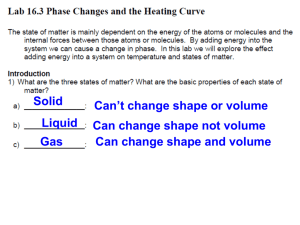
Science Class September 16th , 2015 Warm Up.. • Place your backpacks along the back wall • Grab your notebook, pencil, agenda, glue, scissors, headphones and warm up sheet from the back counter • Complete Wednesday’s warm up now! DO NOT WORK AHEAD! • REMEMBER YOU ARE QUIET IN YOUR SEAT WORKING WHEN THE FINAL BELL RINGS Agenda… • • • • • • Collection of Signed Tests Introduction of New Standard 6.P.2.2 Chromebooks Activity Thermometer Lab (in groups) Foldable for 3 states of matter Bill Nye video Collection of Tests… • Pass in your SIGNED tests now using our procedure now Standard 6.P.2.2 • Turn in your notebook to your 6.P.2 Standard • We will popcorn read Standard 6.P.2.2 now In your notebook… • Go to the next blank page in your notebook and label it 6.P.2.2 States of Matter • Add this to your table of contents also Chromebooks… • Before we begin our lab today, everyone will use their chromebook to learn about thermometers • I will call you back to get your chromebook using our procedure now • Once you are back at your seat: • 1. login • 2. go to www.studyjams.com and wait for directions Studyjams • In a search box type tell temperature • Click on the button Step by Step to complete the activity • 5 minutes to complete • When you finish, record the temperature of the thermometer in your notebook now • Log off and wait quietly for directions Today’s Lab • We are trying to figure out what happens to the atoms that make up matter when it changes state… • To investigate this, we will be investigating water in all 3 states of matter • We will also be investigating how a thermometer works Returning chromebooks • You will return your chromebooks now using our procedure • Return to your seat and wait quietly for directions Expectations during lab… • You will work in an assigned group • Once you move to your group you will not leave your seat unless directed by the teacher • You will take all of your materials to this new seat and stay there for all of the class • Let’s move to our groups now… Lab today: You must be careful with your supplies • We will be using glassware and thermometers • You must use caution today! • Anyone I see playing around or out of their seat will not participate in Science Labs! • I will pass out your supplies now • 1. Glassware and Thermometers Lab today: Everyone write in your notebook • Thermometer Lab • Look closely at your thermometer. The liquid inside is a type of alcohol that’s been dyed red. Lab Today… • Look closely at your thermometer. The thermometer has numbers on it. This is the range of temperatures that the thermometer will measure. • Everyone record the range of temperatures now in your notebook (lowest temp. to highest temp.) • This is a degrees Celsius reading • -20 °C to 110 °C Today’s Lab • Practice reading the temperature in °C by having your eye at the same level as the top of the red liquid. What is the temperature in our classroom? • Each line = 1 °C • Everyone record the temperature now in your notebook • This is the temperature in our room • The liquid is probably between 20 °– 30 °C Today’s Lab • Put your thumb or finger on the red bulb and see if the red liquid moves in the thin tube. • Does the red liquid move? • Why do you think it moved? • It moved up because you are warmer than our classroom temperature Today’s Lab • You will now receive a handout • Your group will determine the temperatures of ice, cold water and room temperature water • You will write the temperature on your handout • You will also draw how you think the atoms are moving in ice cold water, room temperature water and boiling hot water • For safety reasons I will stand near the boiling hot water and call your group back one group at a time Today’s Lab • Now put the thermometer in room temperature water. Keep it in the room temperature water until the liquid stops moving. Record the temperature in °C. • You will do this for ice cold water too Today’s Lab • Now put the thermometer in ice cold water. Keep it in the ice cold water water until the liquid stops moving. Record the temperature in °C. Today’s Lab • I will help you place the thermometer in hot water and you will watch the red liquid. Keep it in the hot water until the liquid stops moving. Record the temperature in °C. Today’s Lab • Does anyone have any questions • You may start now • 15 mins. to complete this lab Collection of supplies • I will call 2 people to bring the supplies to the back counter • Let’s clean up now… States of Matter • • • • • • Now turn your handout over You will see 3 equal sections Label section 1 : Solids Label section 2: Liquids Label section 3: Gases You will copy the following information down now Solids Fact #1: Has a definite volume and shape Add heat Fact #2: Atoms are packed close together. Fact #3: Atoms vibrate a little solid can become a liquid!!! Check it out!!!!! Liquids Fact #1: Has a definite volume, but not a definite shape Add heat Fact #2: Particles touch, but bounce around Fact #3: Take the shape of their container. liquid can become a gas!!! Check this out now!!!! Gases Fact #1: Has no definite volume or definite shape Fact #2: If not contained, gases will spread out to fill the space ComparingAll Three!!! Fact #3: If contained, they Will take the shape of their container. Draw the Atoms! • Watch the atoms on the screen • Now draw the atoms in a solid, liquid, and gas at the bottom of your handout Handout • Fold your handout in half • I will come around an staple it into your notebook now Back to our thermometer lab… • Based on what you know about the way molecules move in hot liquids, explain why the liquid in the thermometer goes up when heated? • When heated, the molecules of the red liquid inside the thermometer move faster. This causes the molecules to spread a little further apart. They have nowhere to go other than up the tube. Back to our thermometer lab… • Based on what you know about the way molecules move in colder liquids, explain why the red liquid in the thermometer goes down when place in ice, cold water? • When the thermometer is placed in ice, cold water, the molecules slow down and their attractions bring them a little closer together bringing them down the tube. Back to our thermometer lab… • Why do you think the tube which contains the red liquid is so thin? • The red liquid is contained in a very thin tube so that a small difference in the movement of the atoms within the liquid will be noticeable. Back to our thermometer lab… • Why does the thermometer have a larger outer tube? • The large outer tube has two purposes—to protect the fragile inner tube and act as a magnifier to help you better see the red liquid. Bill Nye The Science Guy! • https://www.youtube.com/watch?v=VdEmxIn egfQ Exit Ticket: • This is the chemical formula for glucose C6H12O6 • How many carbon atoms are there? • How many hydrogen atoms are there? • How many oxygen atoms are there? Exit Ticket: How did you do? • This is the chemical formula for glucose C6H12O6 • • • • • • How many carbon atoms? 6 How many hydrogen atoms? 12 How many oxygen atoms? 6 Prezi Video • http://prezi.com/rxml51_apd_1/expansionand-contraction/ • • http://www.youtube.com/watch?v=rMg1bmF 5BVo