Introduction and review of Matlab
advertisement

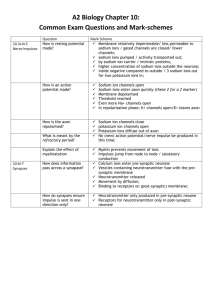
Discussion topic for week 6 : Nerve impulses & ion channels • Potassium channels conduct the K+ ions but reject the smaller size Na+ ions with a selectivity ratio exceeding 1/1000. How do they achieve this feat? Problem of nerve impulses (Nelson, chap. 11, 12) How do we send signals from brain to muscles in milliseconds? The axons, which are the pathways for signals, form leaky cables in a conducting environment (salt solution). Thus compared to a copper wire, sending electric signals across axons is a difficult problem, requiring a novel solution. Besides the leaky cable problem, ions in a salt solution move very slowly due to small D, which greatly reduces the signal transmission speed <x2> = 2Dt, with D~10-9 m2/s, and L = 1 m, gives t ~ 5 x 108 s ~ 16 years! Also ion concentration and energy are quickly dissipated (cf. pulse solution). Even if there were an applied potential difference between the neurons and muscles (solving the latter problem), it wouldn’t help much with the speed vd f f D , ( D kT , Einstein relation) kT eV D eV D 40 10 9 m/s for 1 eV 40 kT L kT kT L L kT L2 t 2.5 10 7 s 1 year vd eV D Action potential basics: Diffusion limits the distance scale of signal propagation to μm or less. The only obvious place where diffusion of ions could make an observable difference is across membranes - ion flow could change the potential difference across the axon membrane. Experimental facts: 1. Na+ concentration is high outside cells and low inside, and vice versa for K+ ions. 2. There are channels on the membrane that, when open, selectively conduct either Na+ or K+ ions. 3. There are ion pumps (called sodium-potassium pump) on the membrane that help to maintain this concentration difference. In each cycle, Na-K pump uses 1 ATP molecule to pump 3 Na+ ions out of the cell and 2 K+ ions in to the cell. Maintenance and propagation of the action potential: 1. Change of membrane voltage opens the sodium channels. 2. Na+ ions flow into the cell, collapsing the membrane potential (−60 mV). 3. This triggers the opening of the potassium channels, while the sodium channels shut down stochastically. 4. K+ ions flow outside the cell, restoring the membrane potential. The potassium channels shut down, returning the system to step 1. 5. This process is repeated along the axon, which propagates the action potential. out in Lessons from squid giant axon: Squid giant axon played a critical role in understanding nerve signals. It has a diameter 1 mm which is 100 times larger than a typical axon’s. Cells have been known to maintain a potential difference with outside for a long time (Galvani vs Volta, ~1800). What is the source of this ΔV? Two observations: 1. Cells are electro-neutral but have more KCl inside 2. K+ is more permeable than ClThus K+ will leak out of the cell until Nernst equilibrium is reached Concentration profiles of K+ and Cl ions across a cell membrane (assuming only K+ is permeable) Corresponding electrostatic potential (from PB eqn.) Nernst potential: V V2 V1 VNernst kT c2 ln ze c1 Realistic case: Inside the cells there are negatively charged impermeant macromolecules whose charge density is equivalent to c_ = 125 mM of excess electrons. The three major ions, Na+, K+ and Cl have the concentrations outside (1): c1Na = 140 mM, c1K = 10 mM, c1Cl = 150 mM From electro-neutrality, the concentrations inside (2) must satisfy c2Na + c2K - c2Cl – c_ = 0 In equilibrium, each permeant species must separately satisfy the Nernst relation with the same potential difference V kT c2 Na kT c2 K kT c2Cl V ln ln ln e c1Na e c1K e c1Cl c1Na c1K c2Cl c2 Na c2 K c1Cl (Gibbs-Donnan relations) Let x c2 Na 1 M c2 K c1K c2 Na c1Na c2Cl c1Cl c1Na c2 Na c2 K 0.01 x 1 M 0.14 c2Cl 1 0.15 0.14 1M x Substitute in the electro-neutrality equation 1 0.021 15 2 x x 0.125 0 x 0.125 x 0.021 0 x 0.21 14 x 14 Inside: c2Na = 210 mM, c2K = 15 mM, c1Cl = 100 mM Donnan potential: V kT 210 ln 25 0.4 10 mV e 140 (Vobs 60 mV ) Observed (expected) concentrations in squid giant axon (in mM): c2 (in) c1 (out) Vnernst (mV) K 400 (15) 20 (10) 75 (-10) Na 50 (210) 440 (140) 54 “ Cl 52 (100) 560 (150) 59 “ All the potentials are different! Hence the cell is not in equilibrium. Na concentration and voltage differs most from the Donnan equilibrium. Ion pumps in membranes actively transport Na+ out and K+ in, and thus maintain this imbalance in concentrations. In one cycle, the Na-K pump hydrolizes one ATP molecule moving 3 Na+ ions out and 2 K+ ions in. Work done for each ion: W(Na+) = e (60 + 54) = 114 meV, W(K+) = e (-60 + 75) = 15 meV Thus the total work done is W = 3 x 114 + 2 x 15 = 372 meV = 15 kT Cf. ATP hydrolysis liberates 19 kT, so only 4 kT is lost as heat. Experimental demonstration of the active ion pumps in the membrane Flux of Na ions out of an axon stops when toxins are introduced Toxins block the pump stopping the transport of Na outside. (Hodgkin-Keynes, 1955) The rate of ATP hydrolysis catalyzed by the Na-K pump as a function of the interior Na and exterior K concentration. Lack of either stops ATP consumption. (Skou, 1957; Nobel 1997) Crystal structure of potassium channel (MacKinnon, 1998; Nobel 2003). Reveals the mechanisms of selectivity and voltage gating Selectivity filter has the right size to bind the K+ ions but too large for the smaller Na+ ions (More details in the video) Voltage gated ion channels: life’s transistors Crystal structure of sodium-potassium pump (Poul Nissen et al. Dec. 2007) F0-F1 ATPase: a molecular rotor in mitochondria 12 nm Exploits the proton gradient to synthesize ATP. The work done by transporting 3 protons across the 11.4 nm d membrane is converted to chemical energy by 3.3 nm synthesizing ATP. 8.2 nm b a c12 9.2 nm Nerve impulses: Response of axons to a weak stimulus: Injecting positive charges in an axon changes the membrane voltage to a more positive value (depolarization). This stimulus spreads along the axon like a pulse solution—its amplitude is dissipated within a few cm. Response of axons to a strong stimulus: Action potential Unless the stimulus is strong enough to change the membrane potential by about 10 mV, it dies down. Above that threshold, it triggers an action potential which propagates along the axon without any loss in amplitude. Time course of an action potential Showing how the membrane potential and the corresponding membrane current change in time. • An initial stimulus opens Na channels • 1-3 inward Na current • K channels open; an outward K current gradually drives the potential back to the resting potential Trigger of action potential in giant squid axon: a) A stimulus of 56 mV depolarizing potential is applied b) Total membrane current c) Inward Na current d) Outward K current Note that the K current starts flowing when the Na current (and the membrane potential) is at a maximum. Both the Na and K channels open in response to changes in the membrane potential (analogous to transistors). Modeling action potential: Analogy with electrical circuits Representing each type of current with a different circuit element, we can write for each one: V V2 V1 I i Ri Vi Nernst Recall that VNernst > ΔV for Na+ and VNernst < ΔV for K+ Capacitive current: Ic dq dV C dt dt jc I c C dV dV CA A A dt dt Cable model Cable equation dV I x ( x) I x ( x dx) jr C A dt dI x dV 2a jr C A dx dt 2a dx (*) V ( x dx / 2) V ( x dx / 2) I x dRx x x Rx A a 2 I x a 2 a 2 d 2V dx 2 dV dx dRx dx a 2 (3 W1m1 conductiv. of axoplasm) Diff. wrt to x and substitute in (*) dV 2a jr C A dt If we assume Ohm’s law for radial conductance: jr (V V 0 ) g tot jr vg tot v V ( x, t ) V 0 a 2 ( Rr 1 / G 1 / Agtot ) d 2v dv 2 a g v C tot A 2 dt dx C A dv a d 2 v v 2 2 g tot dx g tot dt 2axon d 2v dv v 2 dt dx axon a 2 g tot (~1 cm) Linear cable equation CA g tot (~2 ms) Axon’s space and time const. Solution of the cable equation: v( x, t ) e t w( x, t ) 2axone t d 2w dx 2 e t dw 2axon d 2 w dt dx 2 v( x, t ) ce t e dw t e w e t w dt 2axon Diffusion equation with D x 2 4 Dt 4Dt Decaying pulse solution The decaying pulse solution is degraded quickly. Need to give up linearity of g(Na) to sustain the pulse 0 g Na g Na Bv 2 Solution of the linear cable equation compared to the pulse solutions c=1 1 cm D=0.05 m2/s 4cm Solid lines: linear cable equation plotted at x = 1, 2, 3, 4 cm Dashed lines: pulse solutions for the same parameters The radial current density is modified to Nernst jr V Vi Nernst g i0 V VNa Bv 2 i 0 Nernst (V V 0 ) g tot V V0 V0 VNa Bv 2 0 vg tot (v H ) Bv 2 1 0 jr 0 v 0 v1, 2 H H 2 4 g tot B 2 Non-linear cable equation: 2axon d 2v dx 2 dv 1 v(v v1 )(v v2 ) dt v1v2 Leads to a traveling wave solution with velocity: vaxon axon 2v1 v2 1 v2 v1 How do neurons communicate? Structure of GltPh (Glutamate transporter) from Pyrococcus horikoshii