Water pollution
advertisement

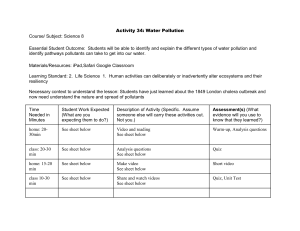
* “George Byron” Private Language School Varna Bulgaria * I. Aims of the lesson II. Theory 1. Water pollution 2. Water pollutants Radioactive pollution Inorganic pollutants Organic pollutants Microorganisms in water Thermal pollution 3. World Water day -22 March. Organizations and strategies preserving water resources III. Control and evaluating 1. Experiments and research 2. Questions 3. Preparing a leaflet - MS Word IV. Summary * To list and differentiate the water pollutants To describe the impact of the pollutants upon the environment and their physiological effect on people To analyze the quality of water by conducting several experiments To summarise the experiment results and make some conclusions about the quality of the water samples To develop a necessity to act sensibly in using and preserving natural resources To know the names of organizations and strategies preserving the water resources * * Water pollution Water is a vital resource, without which our life and industry are unthinkable. It is a significant resource for agriculture, housework, industry and water transportation and source for putting down fires and production of energy. The ecological problems and the sequences are interrelated and can be shown in the following chain: overpopulation→ overproduction→ overconsumption→ large amounts of waste→ depletion of natural resources and their pollution→ diminishing biodiversity→worsen human health * 1. Radioactive pollution- solution of radioactive isotopes • Reasons: as a result of nuclear or radioactive damages, improper storage of • radioactive substances (radioactive substances may be thrown away by nuclear power stations, uranium mines or radiochemical labs, hospitals, etc.) Consequences: The results are: dying or mutating organisms. The worst, by the way, is the fact that the polluted place emits radiation for thousands of years. * Inorganic pollutants Pesticides Herbicides Mineral fertilizers Sediments Inorganic chemicals Acids Compounds of heavy metals Toxic metals * Pesticides - they keep the crops from parasites or stimulate the growth of plants. Pesticides are harmful not only for the parasites, but for humans, too. Source of pollution: agriculture Consequences : Spraying with these substances pollutes the soil as well as water. Physiological effect: Living organisms are poisoned and through underground waters the pesticides leak into the water basins. * Physiological effects: People drinking water which contains higher levels of nitrate suffer shortage of oxygen that reaches any part of the human body through the blood system. The major polluter is agriculture. * These are: acids, salts, compounds of toxic metals such as mercury and lead Consequences : The presence of highly concentrated pollutants may make water impotable, to threaten fish and other water inhabitants, to diminish the crops or worsen the corrosive stability of materials which are near or within the reach of polluted water. The main polluter is industry. Physiological effect: can lead to deposit of heavy metals in blood and inner human organs. Symptoms of intoxication * Insoluble substances (sediments) — These are insoluble substances in the soil which disperse in water mainly by erosion. Consequences : Mud damages water supply systems, dams, water power plants, clogs irrigation networks, moreover, its impact on ecosystems is highly negative (it lessens the penetration of light, which is necessary for photosynthesis of organisms living there and thus the soluble oxygen in water decreases dramatically) Chemicals Sources Health risks Nitrate Agriculture / rain water Risky for new – borns (The blue baby disease) Pesticides Agriculture Cancerous, mutagenic effect, affects the nervous system Petrol products Waste disposal, leakage Cancerous Arsenic Geogenetic factors (soil) Skin disease, cancerous Copper Copper pipes Damages liver Lead Lead pipes Affects the nervous system; may cause retarding effect on physical and mental development of children and babies. Cadmium Galvanic pipes Kidney disease * Petrol/ petrol products Organic waste SWS detergents Organic pollutants Home waste and plastics Oils Dioxins and furans Sources of organic pollution: transportation, industry and human habitats, petrol industry, food industry and pharmaceutical industry Consequences : Organic pollutants are oxidised in a natural way by the oxygen dissolved in the water thus lessening its concentration and worsening the quality of water. Waste waters with organic pollutants usually contain a lot of suspended insoluble substances which diminish the light (necessary for photosynthesis of organisms). Oils and grease form a thin layer on the surface of water and hinder the natural solution of oxygen in it. * Pollution with petrol products is caused by unintentional spills of petrol products in water or soil. Consequences : The results may be life destroying for flora, fauna as well as humans. Petrol spills by damaged tankers can be environmentally harmful because petrol is lighter than water and floats on the surface. These spills may cover thousands of kilometers and stop the oxygen and light for sea animals; cause poisoning and death of the coastal birds, fish, crabs etc. * Consequences: SWS cause serious environmental problems. Industrial and home wash waters pollute water basins and produce destructive effect on river flora and fauna. They disturb the balance in the system which results in dead fish and other river inhabitants. Physiological effect – SWS mainly deoil skin cause allergic reactions to people with highly sensitive skin. Same fungal diseases are observed. * Sources of pollution: All activities of washing machines and vehicles produce waste waters with a lot of oils, grease and other petrol products. Consequences: Oils and grease form a thin layer on the water surface which hinders the natural solution of oxygen in it. * 3.4 Dioxins and furans are sub products of the process of burning or the industrial production of chlorine. They are toxic, difficult to decompose and can be stored up in the body. Dioxins and furans can be transferred by air, water and biological species to great distance and are accumulated in terrestrial and water ecosystems. Consequences: In water environment dioxins and furans are hard to dissolve but they have a very strong ability to deposit and pile in sediments and living organisms. 3.5 Organic waste (animal waste; waste of canning industry; milk industry, etc.) are dissolved in water. The process of dissolving of organic waste uses a great amount of the oxygen in water. * Pollution with some organic materials may result in mechanical pollution of water and soil with insoluble synthetic substances ( for example: plastic bottles, bags, ,etc.) Consequences :It causes problems in the normal development of living organisms. * There are bacteria, viruses, protozoa, parasitic worms, which get into water through drain pipes or untreated human and animal waste. These pollutants cause the death of 14 000 people a day, half of them are children. Physiological effect: They are disease agents ( malignant cholera, hepatitis, typhoid fever, tetanus, etc.) They can be spread by sewage, rivers, atmosphere. http://savewater.uchenici.bg/content/pollution/bacteriological.html * It can be natural (because of hot weather) or artificial (of industrial origin). Sources of pollution: Thermal Power Stations and Nuclear Power stations which release a lot of hot water in rivers and lakes, used for cooling their systems. Consequences: Thermal pollution lessens the amount of dissolved oxygen and this may cause the death of fish and intensive multiplying of pathogenic bacteria; change in the weed biocenosis and bogging of some river parts. The increasing river water temperatures results in many negative consequences from a physical point of view – fogs, erosion, evaporation. * Worlds Water Day was appointed 20 years ago in Rio de Janeiro during a conference. Organisations have been researching global ecological problems for 20 years and have been looking for ways and methods to preserve fresh water. Every year the day is celebrated with a different motto. In 2016 the topic is “Water and Working places” http://www.unwater.org/campaigns/world-water-day/en/ http://www.unicef.bg/en/article/Svetoven-den-na-vodata/716? UNICEF http://www.cap-net.org/world-water-day-2015-learning-pack/ Power Point, quiz, a short video http://www.un.org/waterforlifedecade/ decade of water in UN http://waterbridge.info International Water bridge * 1. Analyze the quality of water by conducting experiments. The students conduct the following experiments, concerning water: smell, opacity. pH, Acid content, alkaloid content, degree of hardness, presence of phenol, nitrates and H2S The students take some water samples from different water basins and label them. The students conduct the experiments and fill the table. The students describe the experiments and the changes. The students summarize the results and make some conclusions about the quality of the water samples. The students present the results and discuss them. * Water samples Proofs Opacity рН Acid content Nitrates Н2S Phenol Reactive substance Description of experiment Change Conclusions/ Summary * Experiment 1: Opacity- The opacity of drinking water can be observer directly. Physical features like opacity can indicate microbiological pollution. Class vessel (0.3l) is full of water. The student holds it against the light. Opacity is defined as follows: clear, slightly opaque, average level of opacity, very opaque. Pay attention to the fact whether hard particles deposit on the bottom of the vessel. Experiment 2: рН pH measures the acid content or alkaloid coutend of water solution (this is also the symbol of acid or alkaloid coutent) The EU rule for drinking water not lower than 6.5 and not more than 9.5 • To measure pH the water temperature should be about 20°C because pH is influenced by temperature • Put a piece of puper in water for 1 – 3 seconds to react and compare it with the colour scale. Experiment 3: Nitrates in water. This can be done only by an experiment, because nitrates are colourless, without any smell or taste. Nitrates in drinking water are dangerous, especially for babies and small children. To define the concentration of nitrates in water, students put a piece of test paper in water for a few seconds, take it out and shake it off. A minute later students compare the colour of the paper against the colour scale. Don`t test when the temperature is below15°C. Please, have in mind that the test is inappropriate for drinking water with chlorine. Experiment 4: Hardness of water (harder water has higher content of minerals - Ca2+ and Mg2+). Pour some sample water in a glass and add a piece of soap. Leave it for 2 minutes, stirring the content from time to time. Experiment 5: Phenol in water Pour some water in a test tube. Add some bromine water. Experiment 6: H2S in water Pour 10ml water in a test tube. You can define the content of H2S because of the smell of rotten eggs or if you add CuSO4 Questions: Please list some of the aims of the Millenium, concerning water preservation? List the environment pollutants and describe the influence of one of them on environment and human health. 3. Homework: Prepare a leaflet by Word concerning the leaflet should be disseminated among relatives and friends. water pollutants. The * Human life depends on water sources and their quality of water consequently avoiding water pollution is our personal responsibility. It’s much easier to avoid water pollution than fighting the consequences of it. If harmful emissions of different productions were caught by special appliances there wouldn’t be acid rains. If cars worked with bio fuels there wouldn’t be any pollution with heavy metals or poisonous gases. If agriculture didn’t use drinking water supplies but rainwater or drop watering we could save enough fresh water. If productions worked with water of a closed cycle the use and pollution would be reduced. If water supply systems were newer and more reliable there wouldn’t be leakages and damages. If the households didn’t use such aggressive washing and cleaning detergents the water basins wouldn’t be polluted with waste waters. If there were more cleaning stations there would be more fresh water. That’s why we should be responsible and sensible in preserving this precious natural source. It’s priceless because our life depends on it.