Limnology
advertisement

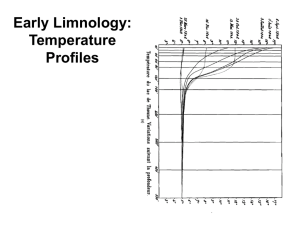
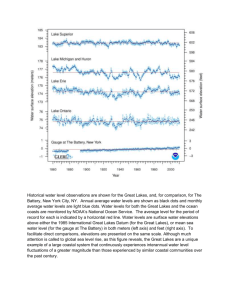
“the oceanography of lakes” Study of the functional relationships and productivity of freshwater communities, as they are regulated by the dynamics of their physical, chemical, and biotic environments Gradually grew to encompass all inland waters Today includes standing water (lentic habitats) as well as running water (lotic habitats) Unique characteristics of water regulate the chemical, physical, and metabolic activities in freshwater systems 1) non-linear relationship between temperature and density 2) high specific heat 3) viscosity-density relationship 4) high surface tension 5) 3 atoms arranged into nonlinear molecule 6) 104.5° angle Structure of Water Molecule Each water molecule can have hydrogen bonds with as many as 4 other water molecules Structure most obvious in ice Creates tetrahedral pattern Lots of space between molecules Low density - it floats Temperature-Density Non-linear Maximum density at 4°C Lower density at higher and lower temperatures Hydrogen bonding and molecular movement Specific Heat Or heat capacity - high for water Amount of heat energy required to raise unit mass 1°C Because of hydrogen bonding Temperature changes occur more gradually in lakes than in terrestrial environs Lakes can buffer climate of nearby land masses Warmer in winter, cooler in summer Viscosity-Density Relation Viscosity - resistance to flow Viscosity of water increases as density increases It doubles as temp decreases from 25°C to 0°C Change not apparent to human eye Profound effect on movements of microscopic plants and animals, and sinking of particles in lakes High Surface Tension Hydrogen bonding interrupted at air-water interface “molecules exert an inward adhesion to the liquid phase” Molecules at surface resist being pulled apart Allows objects that would normally sink in water to be supported on the surface Mercury is the only liquid with a higher surface tension Increases slightly with increased salinity, decreases with increased temperature, addition of organic compounds Limnology (lentic = lakes and ponds; lotic = streams and rivers) Lake Zones Limnology: light Limnology: light WATER CLARITY Causes and significance Causes •Turbidity (Suspended solids) •Plankton blooms •Pollutants Significance •Reduces light penetration •Reduces Photosynthesis •Reduses feeding efficiency of fishes Heating and cooling Sources of heat: 1.Direct solar radiation (most important) 2.Groundwater and springs 3.Ground (minor) Losses of heat: 1.Thermal radiation (primary) 2.Conduction 3.Evaporation 4.Outflow Temperature Cycles & Lake Stratification Most lakes mix during some seasons and become stratified during other seasons. These terms refer to the vertical circulation of water: Mixing = circulation, Stratification = lack of mixing (development of layers) The mixing pattern has a large effect on lake chemistry and the biota Lakes have traditionally been classified according to their annual mixing pattern or mixing regime (amictic, monomictic, dimictic, etc.) Temperate zone Dimictic Lake Stratified Mixing Mixing Stratified Thermal zones in a stratifed lake Metalimnion Mixing and Stratification ESSENTIALS 0F A THERMAL PROFILE: WHAT DOES IT MEAN? Thermal stratification -Effects vertical circulation of water and reduces mixing of deep and surface water •Prevents up welling of nutrients •Concentrates pollution Unstratified lake -Allow nutrients to react shallow water -Nutrients and light allowed for photosynthesis