Properties and Interactions of Matter Worksheet
advertisement

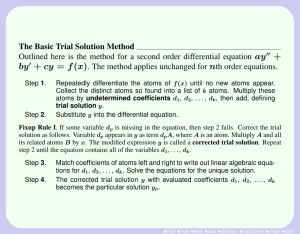
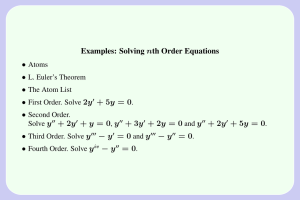
Properties and Interactions of Matter Worksheet Page 5: 1. What is the smallest part of all matter? _______________________________________________________ 4. What are the three basic parts of an atom? __________________________________________________ ________________________________________________________________________________________________________ 5. What electrical charges do these parts have? __________________________________________________ _________________________________________________________________________________________________________ 6. Where are the basic parts found? ______________________________________________________________ _________________________________________________________________________________________________________ 7. Where is most of the mass of an atom found? _________________________________________________ 8. What part of the atom takes up the most space? ______________________________________________ Page 9: 1. How are atoms of the same kind of matter similar? ____________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ 2. What is the atomic number of an atom? ________________________________________________________ _________________________________________________________________________________________________________ 3. How do the number of protons and electrons compare in a normal atom? _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ 5. Why are normal atoms said to be electrically neutral? ________________________________________ _________________________________________________________________________________________________________ 6 & 7 How do you use the periodic table to determine the number of protons in an atom? _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ Page 12: 1. What is an element? ______________________________________________________________________________ ________________________________________________________________________________________________________ 2. What do you know about all of the atoms in the element oxygen? ___________________________ _________________________________________________________________________________________________________ 3. How do oxygen atoms compare to the atoms in another element, Hydrogen? _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ 4. What are two ways that elements can be made? _______________________________________________ _________________________________________________________________________________________________________ 7. What is good about classifying in science? ______________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________ 8. How is it possible that the Periodic Table of the Elements is useful to all scientists, no matter what language they speak? _________________________________________________________________ _________________________________________________________________________________________________________ _________________________________________________________________________________________________________