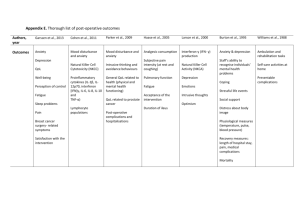
CONSORT 2010 checklist of information to include when reporting a
advertisement

Dear Editors: On behalf of my co-authors, I’m willing to submit you our revised manuscript for your evaluation after we received the comments of peer reviews from Professor Bo Qin (2028395505189727 _comment) and Professor Tarig Merghani (5698284831758952_comment). Its title has been changed to “Effects of G.H.3. on Mental Symptoms and Health-Related Quality of Life Among Older Adults: Results of a Three-Month Follow-Up Study in Shanghai, China” according to the reviewers’ suggestions. When adults enter middle-age, a reduction of intracranial neurotransmitters, cerebral blood flow, cerebral oxygen consumption, and catecholamine can make them more prone to memory and cognitive dysfunction, poor sleep, depression, anxiety, frequent insomnia, fatigue, dizziness, absent-mindedness, response slowly, headaches, tinnitus, and other symptoms and signs associated with aging. These factors can affect the quality of life in elderly people to a large extent, and may even indirectly lead to other chronic diseases, such as hypertension and coronary heart disease. G.H.3. is a nutritional supplement that may provide potential effects on improving general health, well-being, and cognitive function. The current experiment performed was a randomized, double-blind, placebo-controlled study conducted on older adults in China experiencing at least one of the related symptoms/complaints listed above. The objective of this study was to explore the effects of daily use of G.H.3. tablets on relieving mental symptoms and improving health-related quality of life. We certify that we have participated sufficiently in the work to take public responsibility for the appropriateness of the experimental design and method, and the collection, analysis, and interpretation of the data. We have reviewed the final version of the manuscript and approve it for publication. To the best of our knowledge and belief, this manuscript has not been published in whole or in part nor is it being considered for publication elsewhere. Herein, relative information for the submitted manuscript is listed as below: 1. Title: Effects of G.H.3. on Mental Symptoms and Health-Related Quality of Life Among Older Adults: Results of a Three-Month Follow-Up Study in Shanghai, China 2. Corresponding authors: Prof. Rong SHI, Tel: 86-0-13040655160, E-mail address: shirong61@163.com. Prof. Jianyu Rao, Tel: (310) 00-1-794-1567, E-mail address: jrao@mednet.ucla.edu 3. The list of the email addresses for all the co-authors Gang Xu: xugang567@sina.com Zhaochun Cao: 309273734@qq.com Mina Shariff: mshariff@drmresources.com Pingping Gu: Pingping_gu@yahoo.com Tuong Nguyen: tom.nguyen@robinsonpharma.com Tian Zhou: zhoutian_med@163.com Rong Shi: shirong61@163.com Jianyu Rao: jrao@mednet.ucla.edu 1 4. Type of manuscript: Research Article 5. We think that “Effects of G.H.3. on Mental Symptoms and Health-Related Quality of Life Among Older Adults: Results of a Three-Month Follow-Up Study in Shanghai, China” is appropriate for our paper involved in micelle catalysis, which is consistent with the available scope of typical topics mentioned in the section “Guide for authors”. 6. We especially declare here that the abovementioned paper is not simultaneously submitted for publication elsewhere. 7. Information of three potential reviewers is shown as below: (1) Prof. Meiqin Cai Department of nutrition, Shanghai Jiaotong University School of Medicine, Shanghai 200025, P.R. China E-mail: yxyy@shsmu.edu.cn (2) Associate Prof. Xiuhua Shen Department of nutrition, Shanghai Jiaotong University School of Medicine, Shanghai 200025, P.R. China E-mail: srachel@126.com The information of the authors: Gang Xu1, Zhaochun Cao2, Mina Shariff3, Pingping Gu3,Tuong Nguyen3, Tian Zhou4, Rong Shi1,Jianyu Rao5 1. School of Public Health, Shanghai Jiaotong University 2. Jiaxing Community Health Service Center, Hongkou District, Shanghai 3. Department of Research, DRM Resources, 1683, Sunflower Avenue, Costa Mesa, CA 92626, USA. 4. Beijing University of Chinese Medicine 5. Department of Pathology and Laboratory Medicine, in UCLA Medical Center, University of California, Los Angeles The first author: Gang Xu, Zhaochun Cao The corresponding author: Rong Shi, Jianyu Rao Thank you very much for your attention and consideration. Best Regards Yours Sincerely Gang Xu 11-25-2015 2 Point-by-point response to the revised manuscript (Page1) 1. The title has been changed to ” Effects of G.H.3. on Mental Symptoms and Health-Related Quality of Life Among Older Adults: Results of a Three-Month Follow-Up Study in Shanghai, China” 2. We have defined the abbreviations when they were used at the first time. (Page2) 3. We added the Key words: G.H.3.; Mental Symptoms; Health-Related Quality of Life; Older Adults; Depression; Anxiety; Double-blinded; SDS; SAS; SF-36. 4. We have condensed the abstract to a proper size. (Page4-5) 5. In “1. Introduction” section, we added some contents and some references. “Since 1999, China has entered the aging society, by 2010, China's elderly population over 60 years of age has reached to 0.178 billion, accounting for 13.3% of the whole country's population, the figure of the Chinese elderly population will still increase steadily by 8 million per year in the near future[2]”. 6. We also added some contents to demonstrate the innovation/significance of the current study and revised the description of the objective of the study. “As the first G.H.3. randomized controlled trial(RCT) research conducted in Chinese older adults population, the objective of this study was to explore the effects of daily use of G.H.3. tablets on relieving mental symptoms and improving health-related quality of life”. 7. The funding sources of study is School of public health, Shanghai Jiaotong University, The G.H.3. and placebo tablets were both provided by Robinson Pharma, Inc (Orange County, CA, USA). 8. In “2.1 Subjects” section, we added some contents to describe that how many participants were assessed for eligibility and the interpretation of sample size. “All of the participants were men and women over 50 years old who had at least one of the related symptoms/complaints associated with mental symptoms and health-related life quality decline which included: anxiety, inattention, memory decline, insomnia, self-induced fatigue, sleepiness, response slowly, involuntary staring, absent-mindedness, vertigo, dizziness, headaches, tinnitus, palpitations, chest pain, etc. The study excluded 16 individuals who had Alzheimer’s Disease, severe depression, mental illness, cancer of any type, history of any severe disease diagnosis (including severe diabetes, cardiovascular disease, etc.) or currently (within 30 days) taking any prescription or over-the-counter drugs that may affect neuroendocrine function. Then, we also excluded 6 individuals who were eligible but had refused to take part in, so there were 3 totally 100 participants took part in this randomized, placebo-controlled, double-blinded study at the beginning.” “Using random numbers table, the 100 eligible participants who met all the eligibility criteria and provided written, informed consent prior to the study were andomly allocated into the G.H.3. group (Experimental, n=50) or the placebo group (Control, n=50) with simple randomization method, administered either G.H.3. or placebo tablets and were followed up for three months. The determination of sample size was mainly based on the limitations of research period and research funds.” (Page5-6) 9. In “2.2 Intervention”, we described that how many participants dropped out and its reason. “Two subjects in the G.H.3. group dropped out at the first month, because their relatives rejected them to continue taking the tablets, so there were 98 participants been followed once per month.” (Page6) 10. In “2.3 Assessment of outcomes”, we described that which information was obtained from participants’ self-reported, which was obtained from physicians’ examination clearly. “All patients were required to complete a questionnaire which recorded their date of birth, gender, ethnicity, medical history, smoking history, alcohol intake history, medical history, current medication(s), complaints of mental symptoms at the baseline. All of the participants were also required to report their subjective feeling about life quality, depression, anxiety currently(past week) by filling out three measurement scales which included surveys for Self-Rating Depression Scale (SDS), Self-Rating Anxiety Scale (SAS) and a 36-item Short-Form Health Survey (SF-36 scale) at the beginning of being enrolled and one week after they finished to take all of the tablets.” “At the baseline and at the end of the intervention period, physicians evaluated the related mental symptoms (including anxiety, inattention, memory decline, insomnia, self-induced fatigue, sleepiness, involuntary staring, response slowly, absent-mindedness, excessive use of brains, forgettery, vertigo, dizziness, headaches, tinnitus)” 11. We defined symptom improved clearly in the second paragraph of “2.3 Assessment of outcomes” “In order to evaluate the magnitude of symptom improvements among the participants in the G.H.3. group, we figured out the symptom score changes of the same person before and after, if the score of a mental symptom after three-month follow-up reduced by 1 point or more than which at the beginning, it will be defined as symptom improved, otherwise will not.” (Page7and Page 16, 17, 18) 12. We stated the primary outcomes and secondary outcomes clearly in the last paragraph of “2.3 Assessment of outcomes”. “The primary outcomes included the scores of the eight domains in SF-36(PF, RP, BP, GH, VT, SF, RE, MH), the single-item indicator—HT and the side effects. The secondary outcomes included the scores of PCS, MCS, SDS, SAS, the prevalence rate of clinical depression symptom, the prevalence rate of clinical anxiety symptom and the improvement rates on the scores of mental symptoms.” 4 13. We recalculated the score of MCS and PCS with each domain score and the data of Chinese adults model, so the results have been correctly changed (See Table2,3,4 from page 16 to 18) 14. We moved the subsection described some outcomes assessments such as SDS, SAS, SF-36 to ”2.3 Assessment of outcomes”. 15. We converted the original total scores of depression(SDS) and anxiety(SAS) into SDS scores and SAS scores by using original score×1.25(rang 25-100), In order to make the value range of them consistent with other variables in Table 2,3,4, these two kinds of calculation methods are both correct and acceptable. The results of compression were not changed, as well as the conclusions. 16. We calculated the prevalence rates of clinical depression symptom and clinical anxiety symptom using cutoff point of SAS score 50 and SDS score 53 based on the diagnosis criteria in Chinese population. (Page8) 17. We provided age range and median age in the “3.1.1 The baseline demographic characteristics” and the abstract. “Participants were between 50 and 89 years of age, with a median of 62.53 years.” 18. In “3.1.3 Comparisons on depression and anxiety statuses at baseline” section, we compare the clinical depression symptom and the prevalence rates of clinical anxiety symptom between the G.H.3. group and the placebo group at baseline. “The prevalence rates of clinical depression symptom in the G.H.3. group and the placebo group were 56.3% (27/48) and 52.0% (26/50) respectively, with no significant difference( 2 =0.178,p=0.673). And the prevalence rates of clinical anxiety symptom in the G.H.3. group and the placebo group were 41.7% (20/48) and 40.0% (20/50) respectively, with no significant difference either( =0.028,p=0.867).” 2 (Page9) 19. We compared the prevalence rates of clinical depression symptom and clinical anxiety symptom between the G.H.3. group and the placebo group. 20. The third paragraph of discussion in the old version manuscript is a presentation of results, so the main contents of this paragraph have been moved into the “3.2.1 Comparisons on SF-36 scores after the intervention”. 21. In “3.2.2 Comparisons on depression and anxiety after the intervention” section, we compare the clinical depression symptom and the prevalence rates of clinical anxiety symptom between the G.H.3. group and the placebo group after the intervention. 5 “The prevalence rate of clinical depression symptom in the G.H.3. group was 20.8% (10/48), no significant difference was found with the placebo group, 34.0% (17/50) ( =2.127,p=0.145), 2 while the prevalence rate of clinical anxiety symptom in the G.H.3. group was 2.1%(1/48), it was significantly lower than which in the placebo group, 22.0% (11/50), ( =9.040,p﹤0.001).” 2 (Page10) 22. The first paragraph of discussion which repeated the previous introduction were deleted in the new version manuscript. 23. We deleted part of results about the improvement rates on the scores of mental symptoms, only reserved the following parts which is related to the objective of this study in “3.3.3 The improvement rates on the scores of mental symptoms”section. “We found that there were totally six symptoms of which improvement rates exceeding 50%, including insomnia(60.4%), dizziness(60.4%), memory decline(54.2%), self-induced fatigue(54.2%), forgettery(52.1%), inattention(50.0%).” 24. We added a more detail description of the side effects in “3.4 Side effects” in the new version manuscript. “No serious, adverse reaction from taking G.H.3. was found in the study, only one participant(Male, 58 years old) in the G.H.3. group reported that he had felt a slight bloating and nausea occasionally after taking G.H.3., but it was not serious and the symptoms soon relieved, also, there was only one person(Female, 59 years old) in the placebo group reported that she had felt a mild head.” 25. We added some discussion and references in the first paragraph of “4. Discussion” “China has the world’s largest elderly population, meanwhile, the proportion of the elderly population continues to increase, the burden of the elderly related diseases are becoming more and more serious at the same time, for exsample, the results of some meta analyses show that the prevalence of clinical anxiety symptom and clinical depression symptom among the elderly in China has reached to 22.1%[23] and 22.6%[24], respectively. According to sleep disorder, the prevalence has even reached to 47.2%[25], while sleep disorder was reported as a major risk factor of the morning peak phenomenon for an elderly patient with simple systolic hypertension, so improving the sleep quality is extremely essential for older people[26]. Maintaining and improving the quality of life of the elderly has also emerged as a particularly important issue[27].” “The relieving of these symptoms will bring great benefits for the general health and well-being status among older adults population, among all of the related mental symptoms, insomnia symptoms have been got the most obvious improved, as we known, improving sleep quality and reducing the frequency of sleep disorder is potentially very useful for the control of systolic blood pressure level in the morning, and the reduction of the cardiovascular events occurrence among elderly. The improvements of the memory decline, forgettery and inattention will potentially promote the recovery of cognitive function for the older adults and reduce the care giving burden in their families and communities.” 6 (Page11) 26. We used ITT to compare the difference of main outcomes between the two groups and got the very similar results, but the related results were not reported in the paper, so we deleted the paragraph which described the advantage of using ITT in the new version manuscript. 27. We added some discussion focused on the potent effects in relieving the general degrees of depression and anxiety symptoms among older adults “G.H.3. preliminarily exhibited potent effects in relieving the general degrees of depression and anxiety symptoms among older adults. It might also help to reduce prevalence rate of clinical anxiety symptom, but we didn’t observed that G.H.3. tablets could help to reduce prevalence rate of clinical depression symptom. Due to these benefits mentioned above we may expect that the incidence risk of some chronic diseases, such as hypertension and coronary heart disease will be lowered indirectly to some extent.” 28. We adjusted part of the discussion on “5. Limitation” to describe the research limitation more clearly. (Page12) 29. We adjusted the contents of “6. Conclusions” based on the new revised manuscript. “In summary, G.H.3. preliminarily shows positive effects in relieving parts of mental symptoms such as inattention, memory decline, insomnia, self-induced fatigue, response slowly, absent-mindedness, forgettery, dizziness, headaches, etc. and improving the general health and well-being status, promoting the recovery of cognitive function among older adults. Most of SF-36 domains including PF, RP, BP, GH, VT, RE, MH, and HT, as well as the overall quality of life in MCS might get benefit from taking G.H.3. tablets. On the contrary, the average levels of depression and anxiety symptoms were both relieved and the prevalence rate of clinical anxiety symptom was reduced. No serious, adverse reactions from taking G.H.3. were found in this study. These results may assist physicians in determining its clinical applications.” (Page14) 30. We provided a flow diagram to facilitate reading. (Page15) 31. We provided a new table(Table 1) to present the demographic characteristics for the G.H.3. group and the placebo group at baseline. (Page18) 32. We have deleted the old table9-11, and revised a new table(Table 4) which was focused on the magnitude of change. (Page20 to 22) 33. We added some references from page 20 to page 22. 7 (Page23) 34. We defined the acronym “SDS, SAS, SF-36, RP, BP, GH, VT, MH, HT, PCS, MCS” in “Schedule 1 List of abbreviations” which was placed at the end of the new version manuscript. 8 CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Item No Checklist item Reported on page No Title and abstract 1a 1b Identification as a randomised trial in the title Structured summary of trial design, methods, results, and conclusions 2a 2b Scientific background and explanation of rationale Specific objectives or hypotheses 4 4 Interventions 3a 3b 4a 4b 5 5 5 5 4 4-5 Outcomes 6a Sample size 6b 7a 7b Description of trial design (such as parallel, factorial) including allocation ratio Important changes to methods after trial commencement (such as eligibility criteria), with reasons Eligibility criteria for participants Settings and locations where the data were collected The interventions for each group with sufficient details to allow replication, including how and when they were actually administered Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed Any changes to trial outcomes after the trial commenced, with reasons How sample size was determined When applicable, explanation of any interim analyses and stopping guidelines 8a 8b 9 Method used to generate the random allocation sequence Type of randomisation; details of any restriction (such as blocking and block size) Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned 5 5 5 10 Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how If relevant, description of the similarity of interventions Statistical methods used to compare groups for primary and secondary outcomes Methods for additional analyses, such as subgroup analyses and adjusted analyses 5 Introduction Background and objectives Methods Trial design Participants Randomisation: Sequence generation Allocation concealment mechanism Implementation Blinding 11a Statistical methods 11b 12a 12b CONSORT 2010 checklist (for specific guidance see CONSORT for abstracts) 1 2 6-8 No change 5 No stop 5 5 8 Without Page 9 Results Participant flow (a diagram is strongly recommended) Recruitment Ancillary analyses 17b 18 Harms 19 For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome For each group, losses and exclusions after randomisation, together with reasons Dates defining the periods of recruitment and follow-up Why the trial ended or was stopped A table showing baseline demographic and clinical characteristics for each group For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) For binary outcomes, presentation of both absolute and relative effect sizes is recommended Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) Discussion Limitations Generalisability Interpretation 20 21 22 Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses Generalisability (external validity, applicability) of the trial findings Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence Other information Registration Protocol Funding 23 24 25 Registration number and name of trial registry Where the full trial protocol can be accessed, if available Sources of funding and other support (such as supply of drugs), role of funders Baseline data Numbers analysed Outcomes estimation and 13a 13b 14a 14b 15 16 17a 8 11-12 12 10-11 6 6 No stop 15 14 8-10 8-10 10 *We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see www.consort-statement.org. CONSORT 2010 checklist Page 10