Box 8 Standards Chemistry
advertisement

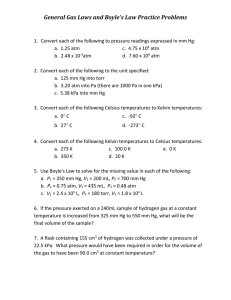
Inside Outside First label letters and numbers very very very freakishly small Second outline the boxes all ten Third in box 5, write Block 7, Nature of Gases, Name, period and Picture of gas in action Picture of Gas in action Vocabulary box 1 1. Charles Law- Temp change Volume change 2. Boyles Law- PV=KT, where K and T are constant 3. Gay-Lussac’s LawV1 / V2 = Whole number 4. Amonton’s Law- T increase means Kinetic Energy increase, P increase 5. Combined Gas Law- Charles and Boyles together PV/T =K 6. Ideal Gas Law- P units pascals, V units m3 T in kelvin, K = rn, r = 8.314J/Kmol n moles PV = nrT Box 8 Standards Chemistry Box B 𝑃1 𝑉2 Box A Charles law A gas is collected and found to fill 2.85 L at 25.0°C. What will be its volume at standard temperature? Convert 25.0°C to Kelvin and you get 298 K. Standard temperature is 273 K Plug into equation 2.85 L = V2 V2 = ?? 298 K 273 K Box C Gay Lussac’s Law ΣVRi / ΣVPi = K Add all volumes divide by all volumes = constant 2 volumes of Hydrogen + 1 volume of Oxygen = 1 volumes of gaseous water Box E Combined Gas Law Chance has a boyle on his face. The boyle exerts a pressure of 2 atm, when it is at a volume of 3 cm3 when he is in the oozarks his boyle is at a volume of 2 cm3, with a pressure of 1 atm. Find the temperature. Oozarks -73c is 200 K (2)(3) = (1)(2) 6(200)= 2T1 T1 200 T1 = ?? 4b- random motion of molecules explains the diffusion of gases. 4a–random motion of molecules and their collisions with a surface create the observable pressure on that surface. 4c- apply the gas laws to relations between the pressure, temperature, and volume of an ideal gas or any mixture of ideal gases. = 𝑃2 𝑉1 Boyles Law 𝑃1 𝑉 = 𝑉2 P1V1 = P2V2 𝑃 2 1 4.40 L of a gas is collected at 100mmHg. What will be its volume after increasing pressure to 200atm? Convert 100mmHg (1atm/760mmHg) = 0.13 atm Plug in values (0.13 atm)(4.4L) = (200 atm) V2 V2 = ??? Box D 𝑃1 𝑃2 Amonton Law 𝑇 = 1 P1T2 = P2T1 𝑇2 A Basketball has a pressure of 31 atm at a temperature of 298K. If Oscar is shooting lights out, he’s on fire, and the ball has a temp. of 325 K find the pressure of the ball. 31 = 298 P2 325 298(P2) = 31(325) Box F Ideal Gas Law PV = nRT Solve for P, V = 2.5 L, n = 3mol R = 8.314 T = 300 P(2.5) = 3(8.314)(300) P = 7482.6 / 2.5 = 2993.04 Box G Copy Questions only Answers in box H 1. Why is a gas easy to compress? 2. List three factors that can affect gas pressure. 3. Why does a collision with an air bag cause less damage than a collision with a steering wheel? 4. How does a decrease in temperature affect the pressure of a contained gas? 5. If the temperature is constant, what change in volume would cause the pressure of an enclosed gas to be reduced to one quarter of its original value? 6. Assuming the gas in a container remains at a constant temperature, how could you increase the gas pressure in the container a hundredfold? Box K Copy Questions only Answers in Box L 14. Briefly describe the assumptions of kinetic theory as applied to gases. 15. Use kinetic theory to explain what causes gas pressure. 16. How is the Kelvin temperature of a substance related to the average kinetic energy of its particles? 17. Convert the following pressures to kilopascals. 18. a. 0.95 arm b. 45 mm Hg 19. A cylinder of oxygen gas is cooled from 300 K (27°C) to 150 K (-123°Q. By what factor does the average kinetic energy of the oxygen molecules in the cylinder decrease? Box I Copy Questions only Answers in Box J 7. How are the pressure and volume of a gas related at constant temperature? 8. If pressure is constant, how does a change in temperature affect the volume of a gas? 9. What is the relationship between the temperature and pressure of a contained gas at constant volume? 10. Write the mathematical equation for Boyle's law and explain the symbols. 11. A given mass of air has a volume of 6.00 L at 101 kPa. What volume will it occupy at 25.0 kPa if the temperature does not change? 12. Explain how Charles's law can be derived from the combined gas law. 13. The volume of a weather balloon increases as the balloon rises in the atmosphere. Why doesn't the drop in temperature at higher altitudes cause the volume to decrease?