DOM research day abstract Yuzhe Yang
advertisement

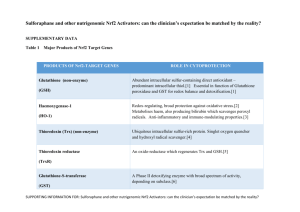
Nrf2 suppression results in growth inhibition and enhances response to chemotherapy and radiation therapy in triple negative breast cancer cells. Yuzhe Yang 1,3, Douglas Yee 1,2,3 1. Pharmacology, University of Minnesota, Minneapolis, Minnesota, United States, 55455; 2. Medicine, University of Minnesota, Minneapolis, Minnesota, United States, 55455; 3. Masonic Cancer Center, University of Minnesota, Minneapolis, Minnesota, United States, 55455. Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a key transcriptional activator that specifically binds to the Antioxidant Response Element (ARE) to initiate expression of various anti-oxidative and anti-inflammation genes to protect cells from oxidative stress. Recently, Nrf2 has been shown to redirect glucose and glutamine into anabolic pathways to promote cell proliferation. Constitutive stabilization of Nrf2 has been observed in many human cancers and confers chemo- and radio-resistance of cancer cells. We examined Nrf2 expression and function in triple negative breast cancer cells (TNBC). mRNA and protein expression of Nrf2 were higher in the TNBC cell lines MDAMB-231 and MDA-MB-436 compared to immortalized breast epithelial cell lines (MCF10A and MCF12A) and estrogen receptor positive breast cancer cell lines (MCF-7 and T47D). shRNA knock-down of Nrf2 in MDA-MB-231 and MDA-MB-436 TNBC cells showed decreased mRNA expression of multiple Nrf2 regulated anti-oxidant (SLC7A11, NQO-1, GCLC, GCLM, SLC7A5, etc) and pentose phosphate pathway genes (G6PD, PGD, ME-1, TKT, TALDO1, PPAT, etc), enhanced basal levels of cellular reactive oxygen species, impaired mitochondrial function as determined by measuring mitochondrial potentials in living cells, and reduced S phase entry. Cells with decreased Nrf2 had reduced cell growth in monolayer and anchorage independent growth assays. In addition, Nrf2 suppression reduced cell migration. Nrf2 down-regulated MDA-MB-231 cells also showed increased response towards ionizing radiation and mitomycin C treatment in clonogenic and soft agar assays. Furthermore, reduced Nrf2 expression decreased the number of stem-like (CD44+/CD24-) cells in MDA-MB-231 cell populations. Thus, Nrf2 regulated many aspects of the malignant phenotype in TNBC and suggest that inhibition of Nrf2 might be a therapeutic target for TNBC.