Electrochemistry - the Science of Oxidation
advertisement

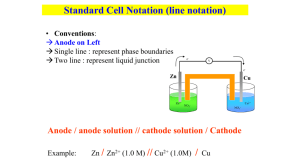
The Nernst Equation Galvanic and Electrolytic Cells 1. Galvanic cells and Electrolysis Cells : in an electrolysis cell, the cell reaction runs in the non--spontaneous direction electrolysis of water 2. Thermodynamics of the relationship of DE and DG, electrical work DG = - n F DE 3. Concentration dependence of the cell potential, the Nernst Equation, the pH meter, the glass electrode, the calomel electrode 4. Adding Half-Cell potentials (problem 12.23a) 5. Disproportionation reactions (problem 12.23b) Galvanic Cells and Electrolysis Cells Galvanic cell: cell reaction runs in the spontaneous direction Electroytic cell: cell reaction runs in the non-spontaneous direction, driven by an applied electrical potential (a voltage) -that is greater than the spontaneous potential negative anode if galvanic cathode if electrolytic positive Ag(s) Cu(s) Cu2+(aq) salt bridge Ag+(aq) galvanic: Eo= 0.+4554 V Cu(s) + 2 Ag+(aq) Cu2+(aq) + 2 Ag(s) electrolytic: E (applied) > Eo cathode if galvanic anode if electrolytic Cathode and Anode in Galvanic Cells and Electrolysis Cells By definition, the cathode is where reduction occurs and the anode is where oxidation occurs. In the galvanic cell, the copper electrode is the anode, silver is the cathode. In the electrolytic cell, the silver electrode is the anode, copper is the cathode. Ag(s) (positive) Cu(s) (negative) Cu2+(aq) salt bridge Ag+(aq) Note that the sign of the potential doesn't change - copper is always negative, silver is always positive. But the designations "cathode" and "anode" change between galvanic and electrolytic cells. The Galvanic Cell This galvanic cell “burns” H2(g) and O2(g) to form water: voltmeter cathode Pt(s) - + anode Pt(s) H2(g) O2(g) salt bridge H3O+(aq) O+(aq) H3 2H3O3+(aq) + 2e- Overall cell reaction: H2(g) + 2H2O 2 H2(g) + O2(g) O2(g) + 4 H3O+(aq) + 4 e- 2 H2O (aq) 6 H2O (l) DEo=+1.229 V The Electrolysis Cell The electrolysis cell runs in the non-spontaneous direction, driven by an external voltage source: power supply: E > DEocell - anode Pt(s) + Pt(s) H2(g) cathode O2(g) salt bridge H3O+(aq) H3O+(aq) 2H3O3+(aq) + 2e- H2(g) + 2H2O Reaction in the electrolysis cell: O2(g) + 4 H3O+(aq) + 4 e- 2 H2(g) + O2(g) 2 H2O (aq) 6 H2O (l) Faraday's Laws of Electrolysis 1. Mass is proportional to electric charge passed through the cell. 2. Equivalent masses of different substances require equal amounts of electric charge passed through the cell. An electrical current of 1 ampere equals one coulomb per second Q (coulombs) = I (amperes) * t (time) 1 mole of electrons has a charge of -96,485 C (the Faraday) moles of e- = (coulombs passed through cell) / 96485 = (I*t ) / 96,485 3 Cu(s) + 2 Au3+(aq) 3 Cu2+(aq) + 2 Au(s) How much gold is deposited if 100 A is passed through this electrolysis cell for an hour? (Ans. 1.24 moles or 245 g) Thermodynamics of Electrochemical Cells DG = DH - TDS (const T) = DE + pDV - TDS (const P) = (qrev + w) + pDV - qrev (reversible, const T) w = - pDV + expansion work wel = - Q DE = - n F DE wel,rev electrical work work = charge * (potential difference) Joules Coulombs Volts DG = wel,rev = - n F DE (const T, P, reversible) Concentration Effects and The Nernst Equation DGo is the standard DG - all solute concentrations must be 1 M and partial pressures must be 1 atm. DG = DGo + RT ln Q - this allows you to calculate DG at non-standard concentrations and partial pressures. DG = - n F DEcell - this is always true; the cell potential is a very fundamental quantity, just like DG DEcell = DEocell - (RT/nF) lne Q - the Nernst equation. It allows you to calculate DG at non-standard conditions Measuring Equilibrium Constants DGo = - RT lne K DG = - n F DEcell - K can be calculated from DGo, which for many compounds can be obtained from thermodynamic tables. - this is always true; the cell potential is a very fundamental quantity, just like DG DEo = (RT/nF) lne K - For reactions in solution, K and can be measured from a standard cell potential. DGo, DEcell = DEocell - (RT/nF) lne Q - The Nernst equation relates DEcell to DEocell The Nernst Equation DEcell = DEocell - (0.0592/n) log10 Q at T = 25oC PbO2(s) + SO42-(aq) + 4 H3O+(aq) + 2 e- PbSO4(s) + 6 H2O(l) Eo=+1.685 V E= Eo - (.0592/2) log10 1 O+]4 [H3 Pt(s) 2-] [SO4 E changes by 0.118 V for each order of magnitude change in [H3O+] or by 0.0296 V for each order of magnitude change in [SO42-]: SO42-(aq) pH = 0 E = +1.685 V pH = 4 E = +1.211 V H3O+(aq) slurry of PbO2(s) + PbSO4(s) pH measurement Standard Hydrogen Electrode: 2 H3O+(aq, 1M) + 2 e- H2(g) + 2 H2O(l) E = +0.241 V - (.0592/2) log10 [H3O+]2 Calomel Electrode: Hg2Cl2(s) + 2 e- = +0.241 + .0592 * pH 2Hg(l) + 2 Cl-(sat’d) Pt(s) Ecell pH Pt(s) H2(g) salt bridge sat’d KCl(aq) H3O+(aq) standard hydrogen electrode E° = +0.000 V paste of Hg(l) and Hg2Cl2(s) KCl(s) calomel reference electrode E° = +0.241 V The Glass Electrode and the pH Meter The glass electrode is non-metallic electrode composed of a special glass that is porous to H3O+. A diffusion potential develops across the membrane in response to a pH gradient. The potential varies by 59.6 mV for each unit change in pH. Ecell = Eocell - 0.0592 V [H+]out2 log10 2 [H+]in2 Ecell = constant + (0.0592 v) * pH Notice that Ecell is uniformly sensitive to pH over 14 orders of magnitude!! Adding Half-Cell Potentials 12.23 a. Calculate the half-cell potential Eo for the half-reaction Cu2+ + e- = Cu+ E3o = ?? using these tabulated potentials: Cu2+ + 2 e- = Cu(s) E1o = +0.340 Cu+ + e- = Cu(s) E2o = +0.522 You can’t simply add the potentials because the numbers of electrons differ. However, you can always add DGo’s: Cu2+ + 2 e- = Cu(s) DG1o = -n F E1o Cu+ + e- = Cu(s) DG2o = -n F E2o Cu2+ + e- = Cu+ DG3o = DG1o - DG2o Adding Half-Cell Potentials (Problem 12.23 a) 12.23 a. Calculate the half-cell potential Eo for the half-reaction Cu2+ + e- = Cu+ E3o = ?? using these tabulated potentials: Cu2+ + 2 e- = Cu(s) E1o = +0.340 Cu+ + e- = Cu(s) E2o = +0.522 DG3o = DG1o - DG2o n3 F E3o = n1 F E1o - n2 F E2o E3o = n1 E1o - n2 E2o n3 Ans: + 0.158 v Reduction Potential Diagrams and Disproportionation same species is both oxidized and reduced 12.23 b. Will Cu+(aq) disproportionate in aqueous solution? 2 Cu+(aq) = Cu(s) + Cu2+(aq) The oxidation-reduction reactions of Cu2+/Cu+/Cu(s) are described on a reduction potential diagram of the form: Cu2+ 0.158 v Cu+ 0.522 v Cu(s) 0.342 v Disproportionation occurs only if the potential to the right of Cu+ is greater than that to the left of Cu+ Cu2+ + e- = Cu+ Cu+ + e- = Cu(s) Reduction Potential Diagrams and Disproportionation (ii) Cu2+ 0.158 v Cu+ 0.522 v Cu(s) 0.342 v Disproportionation occurs only if the potential to the right of Cu+ is greater than that to the left of Cu+ Why? Disproportionation requires that you run this backward Cu2+ + e- = Cu+ and this forward Cu+ + e- = Cu(s) which occurs only if this Eo is more positive than this Eo Ans: yes