WJEC AS Practical Biology SoW (1)

AS Biology Unit One
1.Biochemistry
1.1 .1What are we made of?
1.
2.
3.
4.
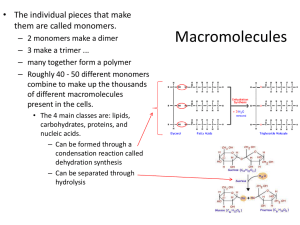
1.1.2 Carbohydrates
1.1.3 Useful carbohydrates 1.
2.
3.
4.
5.
6.
1.
2.
3.
4.
List the main elements found in living organisms.
State that some elements are needed in trace amounts (details not required).
List the key elements which are present as inorganic ions: Mg 2+, Fe 2+, Ca 2+, PO
4
3–
The importance of water in terms of its polarity, ability to form hydrogen bonds, surface tension, as a solvent, thermal properties, as a metabolite.
State the structural difference between α (alpha) and β (beta) glucose.
Describe the formation and breakage of glycosidic bonds in the synthesis and hydrolysis of a disaccharide.
Describe the molecular structure of alpha-glucose as an example of a monosaccharide carbohydrate.
Explain how the structure of glucose relates to its function in living organisms.
Describe the formation and breakage of glycosidic bonds in the synthesis and hydrolysis of a polysaccharide.
Compare and contrast the structure and functions of starch (amylose) and cellulose.
Describe the structure of glycogen.
Explain how the structures of starch and glycogen relate to their functions in living organisms.
Compare and contrast the structure and functions of amylose and cellulose.
Explain how the structure of cellulose relates to its function in living organisms.
1.1.4 Amino acids and proteins
1.1.5 Proteins in action
1.1.6 Lipids
1.1.7 Review
1.
2.
3.
4.
5.
Describe the structure of an amino acid.
Describe the formation and breakage of peptide bonds in the synthesis and hydrolysis of dipeptides and polypeptides.
Explain the term primary structure
Explain the term secondary structure with reference to hydrogen bonding.
Explain the term tertiary structure with reference to hydrophobic and hydrophilic interactions, disulfide bonds and ionic interactions.
1.
2.
3.
1.
2.
3.
Explain the term quaternary structure with reference to the structure of haemoglobin.
Describe the structure of a collagen molecule.
Compare the structure and function of haemoglobin and collagen.
State that lipids (fats and oils) are a range of biological molecules including triglycerides.
Compare the structure of triglycerides and phospholipids.
Explain how the structure of triglyceride and phospholipid and cholesterol molecules relates to their functions in living organisms.
Biochemistry review
Focus on: Numeracy and kinetics (continue in enzymes)
Biomolecule
Protein
Reducing sugars
Nonreducing sugars
Starch
Lipid
Name of test Reagents Instructions Colour change for positive result
Colour change for negative result
The test for Protein
The biuret test is a chemical test used for detecting the presence of peptide bonds. In the presence of peptides, a copper(II) ion forms a violet-coloured complex in an alkaline solution
1. Using a syringe, add 2 cm 3 of protein suspension to a test tube.
2. Using a clean syringe, add 2 cm 3 of sodium hydroxide and shake to mix.
3. Add a small amount of copper sulfate drop by drop, shaking between each addition.
4. Check for any colour change. Do not get confused with the blue colour of the copper sulfate.
5. Repeat the test using a clean test tube and syringes and substituting distilled water for the protein suspension.
6. Check for any colour change.
The test for Lipids
Lipids do not dissolve in water, but do dissolve in ethanol.
This characteristic is used in the emulsion test.
Do not start by dissolving the sample in water, but instead shake some of the test sample with about 4 cm³ of ethanol.
Decant the liquid into a test tube of water, leaving any undissolved substances behind.
If there are lipids dissolved in the ethanol, they will precipitate in the water, forming a cloudy white emulsion .
The test for Starch
The Iodine test is used to test for the presence of starch.
Iodine solution — iodine dissolved in an aqueous solution of potassium iodide — reacts with the starch (starch–iodine complex forming as the iodine fits into the starch helix)
A positive result will produce a purple-black colour.
Carbohydrates can be classed as either :
1. Reducing sugars e.g. all Monosaccharides and some disaccharides.
2. Non reducing sugars e.g. Sucrose and Polysaccharides.
Reducing sugars contain a chemical group which can donate electrons to reduce another chemical.
Aldehyde group
Reducing form of glucose donates electrons
Cu 2+ e
-
Cu 1+
The test for Reducing sugars
• Add a few drops of Benedicts solution to the sample.
• Heat the mixture in a water bath for 10 + minutes.
Observation
• The solution will gradually change from :
Blue Green Brown Orange Red
Explanation
Benedict's solution contains CuSO
4
– this contains Blue Cu 2+ ions
Electrons donated by the Aldehyde group from a reducing sugar slowly reduce the blue Cu 2+ into red Cu 1+
Aldehyde group donates electrons (e-) to reduce the blue Cu 2+ in the solution to red Cu 1+
Some reducing sugars do not have an Aldehyde group , but have a Ketone group which donates electrons in a similar way.
The test for Non reducing sugars
• The Benedicts test gives a negative result with Sucrose and Polysaccharides.
• To detect non reducing sugars the following test should be done.
1.
Heat the sugar with dilute hydrochloric acid. This hydrolyses sugar into smaller units which have Aldehyde groups.
2. The large non reducing is broken into smaller reducing sugars.
3.
Neutralise the acid with Sodium Hydroxide
4. Carry out the Benedicts test as normal.
Result: Blue Cu 2+ Red Cu 1+
Non
Answers
1. Most disaccharides are reducing sugars but sucrose is not. This is because of the way that fructose and glucose are joined, within the molecule, making it impossible to reduce the copper(II) ion in Benedict’s reagent.
2. Sodium hydrogencarbonate is added to the tube before testing with Benedict’s solution to neutralise the acid hydrochloric acid. Benedict’s test will not work in an acid environment .
3. The test on sucrose without any pre-treatment (the control) demonstrates that sucrose will not reduce Benedict’s solution unless it is first hydrolysed into its monosaccharide components.
Is it a carbohydrate?
Add Benedicts’ reagent
Heat
Blue Colour observed
Boil in dilute acid
Neutralise acid
Add Benedicts’ reagent
Heat
Blue Colour observed Not a carbohydrate, possibly a ferret
Green or Red Colour observed
Reducing sugar
Monosaccharide or some disaccharides
Green or Red Colour observed
Non-Reducing sugar
Polysaccharide or some disaccharides
• Colorimetry is method of determining the concentration of a substance by measuring the relative absorption of light (usually visible) with respect to a known concentration of the substance. The instrument by which these measurements are made is called a colorimeter
• Light from a suitable source is passed through a light filter to select the most appropriate wavelength of light, some of which is then absorbed by the solution held in a special glass cuvet (a sort of 'test tube').
• The amount of light absorbed is called, and measured as, the absorbance which is a function of the coloured solute concentration.
• The amount of light that travels through the sample is called, and measured as, the transmission which is a function of the coloured solute concentration.
The semi quantitative basis of the Benedicts test
• the concentration and relative amounts of reducing and non reducing sugars in a solution can be estimated by the Benedict's test.
To look at concentration
• the
quicker the colour change
from Blue Cu 2+ to Red Cu 1+ the stronger the sugar concentration – there will be more Aldehyde groups donating electrons to reduce the Cu 2+ .
To look at relative amounts of reducing/non reducing sugars
• if you carry out the reducing sugar test and get a slow colour change and then carry out the non reducing test and get a rapid colour change...
... what does this suggest about the relative amounts of reducing and non reducing sugars in the sample?
Describe how the concentration of glucose in a solution may be determined using colorimetry
• Use known concentrations of glucose (0.2, 0.4, 0.6, 0.8, 1.0 mol dm -3 etc)
1. Heat with Benedicts solution
2. Using the same volumes of glucose solutions each time
3. Using excess Benedicts
4. Benedict’s changes to green / yellow / orange / brown / red colour
5. Remove precipitate by filtering
6. Zero the colorimeter using water
7. Use a filter
8. Read the absorbance
• Less absorbance of filtrate = more sugar present
• Plot a calibration curve by plotting absorbance against glucose concentration
• Use the reading of the unknown glucose solution and read off graph to find concentration
Describe how the concentration of glucose in a solution may be determined using colorimetry