The Earth & Climate Change
advertisement

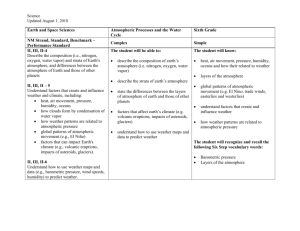
Atmospheric & Oceanic Processes Lecture 2: Characteristics of the atmosphere Fresh snow… Feb/06/2016 Some parameters of Earth. Earth’s rotation rate Surface gravity Earth’s mean radius Surface area of Earth Area of Earth’s disc Mass of Earth Ω g a 4πa2 πa2 ME 7.27 × 10−5s−1 9.81 ms−2 6.371 × 106 m 5.09 × 1014 m2 1.27 × 1014 m2 5.972 × 1024 kg By Newton’s law of universal gravitation, force exerted by mass ME on mass m is: Fg = -(GMEm/r2)(r/r) where G (= 6.67408 × 10-11 m3 kg-1 s-2) is the universal gravitational constant, r is the distance vector measured from the center of mass of ME, the earth, to an air mass parcel m of the atmosphere, i.e. r = |r| = a + z, where z is the height of the atmospheric air parcel measured from the mean sea level (where z=0). Then, earth’s gravity g (i.e. acceleration due to gravity) is: g = (Fg/m) = -(GME/r2)(r/r) -(GME/a2)(r/r) 9.81 m s-2 (r/r) where we have made use of the fact that the atmosphere is “thin”… Most of the atmosphere is contained in a “shell” of about 10 km high (see HW1, & below), which is very thin compared to the radius of the earth Figure: The thinness (to scale) of a shell of 10km thickness on the Earth of radius 6370 km Atmospheric wind is never completely blocked by topography Ocean current is confined to basins A north-south section of topography relative to sea level (in meters) along the Greenwich meridian (0◦ longitude) cutting through Figure below (red line). Antarctica is over 2km high, whereas the Arctic Ocean and the south Atlantic basin are about 5km deep. Note how smooth the relief of the land is compared to that of the ocean floor. World relief showing elevations over land and ocean depth. White areas over the continents mark the presence of ice at altitudes that exceed 2 km. The mean depth of the ocean is 3.7 km, but depths sometimes exceed 6 km. The thin white line meandering around the ocean basins marks a depth of 4 km. TABLE 1.2. Some of the most abundant atmospheric constituents. Chemical species Molecular weight (g mol−1) Proportion by volume N2 O2 Ar H2O (vapor) CO2 28.01 32.00 39.95 18.02 44.01 78% 21% 0.93% ∼0.5% 380 ppm Water vapor (H2O) is mainly from ocean evaporation. CO2 are due to photosynthesis and respiration, ocean-atmosphere exchange & anthropogenic activities. H2O & CO2 are important in radiative transfer (the passage of radiation through the atmosphere), because they strongly absorb and emit in the infrared, the region of the spectrum (wavelengths about 10 μm) at which Earth radiates energy back out to space. H2O & CO2 are present in very small concentrations, and so are sensitive to anthropogenic activities. Atmospheric CO2 concentrations observed at Mauna Loa, Hawaii (19.5◦ N, 155.6◦ W). Note the seasonal cycle superimposed on the long-term trend. The trend is due to anthropogenic emissions. The seasonal cycle is thought to be driven by the terrestrial biosphere: net consumption of CO2 by biomass in the summertime (due to abundance of light and heat) and net respiration in wintertime. Over the course of Earth’s history, CO2 levels have greatly fluctuated; they are different in warm as opposed to cold periods of Earth’s climate. For example: 20,000 years ago, 30 million years ago: 220 million years ago: CO2 ~ 180 ppm; (cold period: last glacial maximum) CO2 ~ 600 ppm; (very warm period) CO2 ~ 2,000 ppm; (very very warm) If the CO2 curve shown above continues to rise, then by year 2100, CO2 ~ 600ppm—not seen since 30 million years ago, a period of great warmth in Earth history. We focus on the lowest 50km of the atmosphere, wherein the mean free path of atmospheric molecules is so short and molecular collisions so frequent that the atmosphere can be regarded as a continuum fluid; it satisfies gas law: p = ρRT where p is pressure, T absolute temperature in K, and the gas constant R = 287 J kg−1K−1. Note: For dry air, any 2 of p, ρ, and T is sufficient to define its state; If p, then ρ, for T = constant air is compressible; If T, then ρ-1, for p = constant air expands. Some atmospheric numbers “Standard” Pressure, temperature & density Properties of dry air at standard temperature and pressure Gas constant for water vapor Rv 461.39 J kg−1 K−1 For a mixture of gases, the gas law is applied individually to the different components; e.g. dry air & water vapor: Atmospheric air = dry air + H2O = moist air Let V = some volume of moist air, then partial densities are: ρv = mass of H2O per unit V and ρd = mass of dry air per unit V, Then: Partial pressure of H2O = e, is the pressure that H2O would exert, at the same T, if it alone occupies V; Similarly: Partial pressure of dry air = pd, is the pressure that the dry air would exert, at the same T, if it alone occupies V. Apply the gas law individually to dry air & H2O: e = ρvRvT & pd = ρdRdT Total pressure is then: p = pd + e (a) (b) e = ρvRvT (partial pressure of vapor) pd= ρdRdT (partial pressure of dry air) p = pd + e (total pressure of vapor & dry air) Air over water in a closed box at temperature T. At equilibrium the rate of evaporation equals the rate of condensation. The air is saturated with water vapor, and the pressure exerted by the vapor is e = es, the saturated vapor pressure. On the right we show the mixture comprising dry ‘d’ and vapor ‘v’ components. es is higher for warmer T So if the air in (b) above is cooled, then water vapor must condense out to return to the water below, and a new lower es in the “atmosphere” above is established at the lower T Warm water is poured into a carboy to a depth of 10 cm or so, as shown on the left. We leave it for a few minutes and throw in a lighted match to provide condensation nuclei: small particles on which the vapor can condense. We rapidly reduce the pressure in the bottle by sucking at the top. The adiabatic expansion of the air reduces its temperature and hence the saturated vapor pressure, causing the vapor to condense and form water droplets, as shown on the right. Condensation nuclei in atmosphere: sulfate aerosols, dust, smoke from fires, ocean salt… A photograph of the sound barrier being broken by a US Navy jet as it crosses the Pacific Ocean at the speed of sound just 23 m above the ocean. Condensation of water is caused by the rapid expansion and subsequent cooling of air parcels induced by the shock (expansion/compression) waves caused by the plane outrunning the sound waves in front of it. When we see “mist” the partial water-vapor pressure e has reached the saturated vapor pressure es To reach higher es, we need to raise the T… (b) T profile for the ‘‘US standard atmosphere’’ at 40◦ N in December. (a) es (in mbar) as a function of T in ◦C (solid curve) Clausius-Clapeyron Relation: es A exp(T) T in oC, A = 6.11 hPa, = 0.067 oC-1 -10oC (a) 3hPa (b) Temperature (K) -30 -10oC • Vapor is in the lowest few km’s • Tropics are more moist than poles • Precipitation when moist air cools by convection, forcing H2O concentrations back to saturation at the lower T • Warm climate is more moist The Clausius-Clapeyron Relation: es A exp(T) T in oC, A = 6.11 hPa, = 0.067 oC-1 has important implications for global warming. It predicts a strong increase of near-surface water vapor content with temperature (about 6~7% per K of warming) So, if T increases by 1K, does the CC-relation mean that precipitation also increases by 6~7%? We will see that precipitation indeed increases as the planet warms, but the increases is less 1~3%... Why? Before this can question can be addressed, we need to digress and first study some basic properties of the atmosphere… Definitions and nomenclatures “Top” of atmosphere, e.g. pressure ~ 1 hPa Wind or ocean current velocities: u: zonal (west to east) v: meridional (south to north) w: vertical (>0 upward) Land orography Coordinate axes: x: zonal (west to east) y: meridional (south to north) z: vertical (>0 upward, =0 at mean sea level) Sea surface Mean ocean depth Ocean’s bottom ZT(x,y) Hydrostatic balance If the atmosphere were at rest, or static, then pressure at any level would depend on the weight of the fluid above that level. This balance is called hydrostatic balance. FT = − (p + δp)δA z Fg = −gM FB = pδA Mass of the cylinder is Gravitational force Pressure force at top face Pressure force at bottom face M = ρδAδz Fg = −gM = −gρδAδz FT = −(p + δp)δA FB = + pδA Net force Fg + FT + FB = 0 δp + gρδz = 0: ∂p/∂z + gρ = 0 Hydrostatic Equation An Application of the Hydrostatic Equation: ∂p/∂z = −gp/RT (using hydrostatic equation ∂p/∂z = -gρ and the Gas Law: p = ρRT R = 287.05 J kg-1 K-1 = 287.05 m2 s-2 K-1) Assume temperature is constant = T0, then we have an “isothermal” atmosphere ∂p/∂z = −gp/RT0 = −p/Hs; Hs = RT0/g = the Scale Height So, p(z) = ps exp(−z/Hs). or, z = Hs ln(ps/p). If we choose a representative value T0 = 250 K, then Hs = 7.31 km. Therefore, for example, in such an atmosphere p is 100 hPa, or one tenth of surface pressure, at a height of z = Hs × (ln 10) = 16.83 km. The isothermal assumption is actually not too bad, if we choose an appropriate Hs, as shown in the figure at right, which shows observed profile of pressure (solid) plotted against a theoretical profile (dashed) based on the above equation for p(z) with Hs = 6.8 km. For isothermal atmosphere (from previous slide), p(z) = ps exp(−z/Hs). The corresponding density is then, from the Gas Law: ρ(z) = [ps/(RTo)].exp(−z/Hs). Mass of the atmosphere (per unit m2) can be estimated: 𝑧 𝜌𝑑𝑧′ = (ps/g).[1 - exp(−z/Hs)]. 0 Set z = ∞: Total mass/m2 = (ps/g). Because of the exponential decay with height, most of the mass of the atmosphere is very near the surface. Thus 80% of it is below z80% where: Therefore, 1 - exp(−z80%/Hs) = 0.8 z80% = -ln(0.2)Hs 1.6 × 7.3km 12 km; i.e. about 80% of the mass of the atmosphere is below an altitude of about 12 km, i.e. within the troposphere. Pressure gradients Homework #2 All HWs are due in 2 weeks from the time they are distributed. 1. Derive or write down the formula for the Rossby number Ro . 2. Explain how the magnitude of Ro can describe if a given atmospheric or oceanic flow can “feel” the earth’s rotation. 3. Explain how the behaviors of atmospheric or oceanic flow can be very different on equator and over the poles? 4. Do these different behaviors explain why typhoons or hurricanes do not develop near the equator? Explain as best as you can. 5. Derive or write down the formula for the hydrostatic equation? 6. Explain why for large-scale atmospheric or oceanic flow the hydrostatic equation is valid? 7. Then also explain why over the hill near the front gate of National Central University, the hydrostatic equation is not likely to apply to describe the wind that blows over the hill? 8. What are the approximate densities of air and water? 9. On earth’s surface, we experience the weight of air some 10 km high. Estimate how deep into the ocean do we need to dive in order to experience the same pressure. 10. What is the saturation vapor pressure es at 15oC? 11. Then show that the corresponding ρv = mass of water vapor per unit volume at 15oC is equal to 0.0126 kgm−3. This is the maximum amount of water vapor per unit volume that the atmosphere can hold at this temperature.