Lab 1: Osmosis & Diffusion Introduction: Kinetic energy, a source of

Lab 1: Osmosis & Diffusion
Introduction: Kinetic energy, a source of energy stored in cells, causes molecules to bump into each other and move in new directions. Diffusion is the result of this contact. Diffusion is the random movement of molecules to an area of lower concentration from an area of higher concentration.
Osmosis is a type of diffusion. This is the diffusion of water through a selectively permeable membrane from a region of higher water potential to a region of lower water potential. Water potential is the measure of free energy of water in a solution. A living system also contains an active transport to create movement of particles like ions that move against their concentration gradient.
The energy source ATP is used during this process to move the particles across the cell membrane.
This experiment takes place to measure the diffusion of small molecules through dialysis tubing. This tubing acts as a selectively permeable membrane, allowing larger molecules to pass through, but slowly. Dialysis is the movement of a solute through a selectively permeable membrane.
When the two solutions on either sides of the membrane are equal and no net movement is detected, the solutions are isotonic. This means that the solutions have the same concentration of solutes. If two solutions differ in the concentration of solutes that each has, the one with more solute is hypertonic. The solution that has less solute is hypotonic.
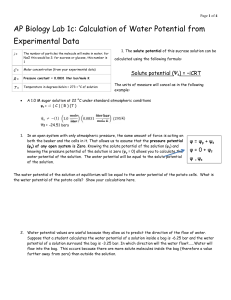
Water potential is predicting the movement of water into or out of plant cells. It is abbreviated by the
Greek letter psi and has two components; a physical pressure component, pressure potential, and the effects of solutes, solute potential. Water always moves from an area of high to low water potential.
The equation is water potential equals the sum of pressure potential and solute potential.
In a plant cell, turgor pressure is necessary. This is a pressure available to plants in a hypotonic environment. Turgor pressure gives plants their structure and strength. When a plant cell is in an isotonic solution, the turgor pressure decreases, causing wilting in the plant structure. In hypertonic solutions, plants plasma membrane shrinks away from the cell wall, an action termed plasmolysis.
Hypothesis: Diffusion and osmosis occur between different molar solutions until the solutions are isotonic, effecting the turgor pressure of plant cells.
Materials: Lab 1A – The materials used in conducting this experiment are as follows: one 30cm strip of dialysis tubing (presoaked), distilled water, 15%glucose/1%starch solution, 250mL beaker,
Iodine Potassium Iodide solution, glucose Testape, and string.
Lab 1B – The materials used in conducting this experiment are as follows: six presoaked strips of dialysis tubing, distilled water, 0.2M, 0.4M, 0.6M, 0.8M, and 1.0M solutions of sucrose, six 250mL glass beakers, string, and an electronic balance.
Lab 1C – The materials used in conducting this experiment are as follows: six 250mL glass beakers, a potato, a core borer, a knife, distilled water, , 0.2M, 0.4M, 0.6M, 0.8M, and 1.0M solutions of sucrose, string, a ruler, and an electronic balance.
Lab 1D – The materials used in conducting this experiment are as follows: graph paper, pencil, a ruler, a calculator, and colored pencils.
Lab 1E – The materials used in conducting this experiment are as follows: a light microscope, microscope slide, cover slip, distilled water, NaCl solution, paper, pencil, and onion skin.
Procedure: Lab 1A : Obtain a 30cm piece of dialysis tubing that has been presoaked in distilled
water. Tie off one end securely. Open the other end of the dialysis tube and insert 15mL of
15%glucose/1%starch solution. Tie off the other end of the bag, leaving room for expansion. Record the color of the solution within the bag. Test the 15%glucose/1%starch solution for the presence of glucose using Testape. Fill a 250mL beaker with distilled water and add approximately 4mL of
Lugol’s solution (IKI) to the distilled water. Test this solution for the presence of glucose as well with the Testape. Record the results in the data table. Immerse the bag in the beaker of solution. Let this stand for approximately 30min, or until distinct coloration is observed. Record final colors of solutions in the bag and in the beaker. Test both solutions once more for the presence of glucose with the Testape strips.
Lab 1B: Before starting this lab, wash your hands. Obtain six 30cm dialysis strips that have been presoaked in distilled water. Tie off each end securely. Pour approximately 25mL of each sucrose molar solution into its respective bags (that should be labeled, but not on the tubing itself). Tie off the other ends securely with string, careful to get any air bubbles out and leaving room for expansion.
Rinse off each bag and blot off the water. Weigh and record the initial mass of the dialysis bags in the data table. Fill six 250mL glass beakers 2/3 full of distilled water and label each beaker with its respective bag’s molarity of sucrose. Immerse each bag into the distilled water. Allow this to stand for thirty minutes. Remove each bag, blot the sides to get off extra solution and weigh and record mass in grams each bag and determine the mass difference and percent change in mass. Next, compare the group percentages to the class.
Lab 1C : Pour 100mL of the assigned sucrose solutions into their 250mL beakers (pre-labeled).
Obtain a large potato. Using a core borer, take 24 samples out of the potato, and measure each in centimeters so that they are all equal in length (use the knife to slice off ends). Make sure not to leave any skin with the samples. Place these cores in a covered beaker until an electronic balance can be obtained. Determine the mass of four cores at a time, placing the four in their sucrose solutions.
Record this data for each of the six beakers. Allow these potato samples to sit immersed in the solutions overnight, covered. Remove the cores, blot off excess solution, and weigh the samples, recording the mass in the data table. Determine the mass difference, the percent change in mass and the class average percent change in mass. Graph the increase and decrease in mass of the potato cores according to the molarity of the solutions they were placed in on graph 1.2.
Lab 1D : Using paper, a pencil, and a calculator determine the solute potential of the sucrose solution, the pressure potential, and the water potential. Also, obtain graph paper and graph the values given for the zucchini percent change in mass and molarity of sucrose solutions in the graph 1.3.
Lab 1E : Prepare a wet mount slide of onion skin. Observe under a light microscope and sketch what you see. Add a few drops of the NaCl solution, observe, and sketch what you see there as well.
Data: Table 1.1 The presence of glucose in beaker and bag solutions
Bag
Initial Contents
15%glucose/1%starch solution
Initial
Solution
Color
Clear
Final
Solution
Color
Midnight blue
Initial
Presence of
Glucose
Final
Presence
of Glucose
+ +
Beaker Water and IKI indicator Amber Amber -
Table 1.2 Dialysis Bag Results: Individual Data
+
Contents in Bag
Distilled water
Initial Mass
28 g
Final Mass
28g
0.2M sucrose
0.4M sucrose
0.6M sucrose
0.8M sucrose
26.9g
27.0g
28.4g
28.2g
28.5g
29.4g
32.6g
32.0g
1.6g
2.4g
4.2g
3.8g
1.0M sucrose 29.9g 34.8g 4.9g
Bags immersed @ 12:01pm, removed at 12:31pm.
Table 1.3 Dialysis Bag Results: Class Data
Group 1 Group 2 Group 3
Distilled water 0%
0.2M sucrose 5.95%
0.4M sucrose
0.6M sucrose
8.89%
14.79%
0.8M sucrose
1.0M sucrose
13.48%
16.39%
0%
4.01%
8.63%
-8.06%
15.19%
7.29%
.71%
5.41%
8.89%
10.69%
12.33%
15.13%
Mass Difference Percent Change
in Mass
0g 0%
5.95%
8.89%
14.79%
13.48%
16.39%
Group 4
.38%
1.75%
8.42%
9.61%
15.70%
12.50%
TOTAL
1.09%
17.12%
34.83%
27.03%
56.70%
51.31%
Class Average
0.27%
4.28%
8.71%
6.76%
14.10%
12.83%
Contents in
Beaker
Initial Mass Final Mass
Distilled
water
3.4g
0.2M sucrose 3.4g
0.4M sucrose 6.2g
0.6M sucrose 6.3g
0.8M sucrose 6.2g
1.0M sucrose 6.0g
4.1g
Distilled
water
0.2M
sucrose
0.4M
sucrose
0.6M
sucrose
0.8M
sucrose
1.0M
sucrose
20.59%
2.94%
-14.52%
-23.81%
-29.03%
-28.33%
Mass
Difference
.7g
Percent
Change in
Mass
20.59%
3.5g
5.3g
4.8g
4.4g
4.3g
Group 1 Group 2
.1g
0.9g
1.5g
1.8g
1.7g
Group 3
2.94%
-14.52%
-23.81%
-29.03%
-28.33%
Group 4
13.33%
-3.39%
-18.64%
-26.23%
-26.32%
-29.31%
14.81%
-7.69%
-15.38%
-23.08%
-26.92%
-30.78%
15.00%
-2.94%
-17.65%
-18.18%
-20.59%
-24.24%
Class
Average %
Change
15.93%
-2.77%
-16.55%
-22.83%
-25.72%
-28.17%
Table 1.4:
Potato
Core:
Individua
l Data
Table 1.5:
Potato
Core
Results:
Class
Data
TOTAL
63.73%
Class
Average
15.93%
-11.08%
-66.19%
-91.30%
-102.86%
-112.66%
-2.77%
-16.55%
-22.83%
-25.72%
-28.17%
Questions:
Which substance(s) are entering the bag and which are leaving the bag? What experimental
evidence supports your answer? Iodine Potassium Iodide is entering the bag because the indicator’s color is concentrated in the bag when the IKI started only in the beaker. Glucose and water left the bag, the evidence was the Testape color.
Explain the results you obtained. Include the concentration differences and membrane pore
size in your discussion. The results simply state that the water, glucose, and IKI were small enough molecules to pass through the selectively permeable membrane. The starch didn’t leave the beaker because its color was amber and the starch molecule was much too large to pass through the selectively permeable membrane.
Quantitative data uses numbers to measure observed changes. How could this experiment be modified so that quantitative data could be collected to show that water diffused into the
dialysis bag? Quantitative data would include the initial and final percent concentrations of the glucose, therefore showing the concentration of water by the change of percent in glucose. The movement would be noticeable in the concentration because it is known that water moves from high water potential to a low water potential.
Based on your observations, rank the following by relative size, beginning with the smallest:
glucose molecules, water, IKI, membrane pores, and starch molecules. The smallest substance was IKI, followed by water, glucose, the membrane pores, then the starch molecules.
What results would you expect if the experiment started with a glucose and IKI solution inside
the bag and only starch and water outside? Why? Based on the size of the molecules, the glucose and IKI would move out of the bag, the water in, and the starch left in the beaker again.
Explain the relationship between the change in mass and the molarity of sucrose within the
dialysis bags. These two things are directly proportional. As the mass increases, so does the molarity.
Predict what would happen to the mass of each bag in this experiment if all the bags were
placed in a 0.4M sucrose solution instead of distilled water. Explain your response. These are inversely proportional because whenever the sucrose molarity inside the bag is more concentrated, it will become more dilute and vise versa. The solutions will reach equilibrium somewhere between the two concentrations.
Why did you calculate the percent change in mass rather than simply using the change in
mass? The differences in mass don’t deal with the proportional aspect of the solutions, making the real results less accurate. The percent was calculated to give the exact difference, along with considering the quantities of solution.
A dialysis bag is filled with distilled water and then placed in a sucrose solution. The bag’s initial mass is 20g, and its final mass is 18g. Calculate the percent change of mass, showing
your calculations in the space below. 18g(final mass) – 20g(initial mass)=-2/20g(initial mass) x
100, which gives you a 10% change of mass.
If a potato is allowed to dehydrate by sitting in the open air, would the water potential of the
potato cells decrease or increase? Why? The water potential of the potato would decrease because water moves from a high water potential region to a low potential region, and a dehydrated potato cell is hypertonic in comparison with the environment, forcing water to come into the cell. The moving in part shows that the potato cell had a low water potential.
If a plant cell has a lower water potential than its surrounding environment, and if pressure is equal to zero, is the cell hypertonic or hypotonic to its environment? Will the cell gain water
or lose water? Explain your response. If the plant cell has lower water potential, that means the water will come into the cell, the cell is hypertonic to its environment. This cell will gain water because water follows its concentration gradient.
In figure 1.5, the beaker is open to the atmosphere. What is the pressure potential of the
system? The pressure potential in this figure is equal to zero.
In figure 1.5, where is the greatest water potential? The greatest water potential is within the dialysis bag.
Water will diffuse__the bag. Why? Water will diffuse out of the bag because the highest water potential is inside the bag, forcing the water out.
Calculate solute potential of the sucrose solution in which the mass of the zucchini cores does
not change. Show work. Y s
=iCRT therefore Y s
=(1)(1.0mole/liter)(0.0831 liter bar/mole degrees
K)(295 degrees K) Y s
=-24.51 bars.
Calculate the water potential of the solutes within the zucchini cores. Show work. Y =Y s
+Y p
so
Y =0+-24.51 , Y =-24.51bars
What effect does adding solute have on the solute potential component of that solution? Why?
Adding solute to a solution would increase the solute potential and decrease the water potential.
Consider what would happen to a red blood cell placed in distilled water: Which would have
the higher concentration of water molecules?The distilled water would have the higher concentration of water molecules.
Which would have the higher water potential? The red blood cell would have the higher water potential.
What would happen to the red blood cell? Why? The red blood cell would take in a lot of water and might lyse due to pressure inside. This is a possibility because animal cells have no tolerance under hypotonic situations.
Describe the appearance of the onion cells. The onion cells appear to have great turgor pressure, spread out, thick and bright in the inside. The cell walls were very defined and it was clear where one cell ended and another began.
Describe the appearance of the onion cells after the NaCl was added. The plasma membrane shriveled from the cell wall, causing plasmolysis. The cells looked wrinkly or weak. The turgor pressure dropped tremendously.
Remove the cover slip and flood the onion with fresh water. Observe and describe what
happened. The onion cells were again hypertonic to their environment, and gathered water, increasing in turgor pressure and restoring themselves to the normal state of being.
What is plasmolysis? Plasmolysis is the separation of the plasma membrane from the cell wall in a plant cell.
Why did the onion cell plasmolyze? The environment became hypertonic to the cell and the water left the cell running with its concentration gradient due to the NaCl. With all the water leaving the cell, it shrank, leaving behind its cell wall.
In the winter, grass often dies near roads that have been salted to remove ice. What causes
this to happen. The salt causes the grass’s environment to become hypertonic, and the water leaves the plant cells, causes withering and eventually death of the plant.
Error Analysis: Lab 1A : One possible source of error could be the tightness of the string that tied off the dialysis tubing. If there was a leak or a break in the dialysis tubing, all of the data would be off.
Lab 1B: A possible source of error in this lab could have been in the first step. If the handler of the dialysis tubing did not wash their hands and accidentally touched the sac part of the tubing, the oils from their hands could have blocked some of the pores on the tubing, distorting the data.
Lab 1C: A piece of potato skin could have been left in the beakers along with the potato. This causes problems in the data tables. Another possible source of error could be that the students did not pat dry the potato sample well enough causing drops to be left on the electronic balance, tarring it incorrectly, causing all other data to be off slightly.
Lab 1D : Simple mathematical errors always occur, so there is always room for simple algebraic
mistakes in this section of the lab.
Lab 1E : If the wet sample was not prepared correctly, or the salt solution added to fast not giving the cells time to react, this lab would have different results. The sources of error also include the possible concept that the onion cells might have dried out by the time the observer got around to sketching.
This could cause error in observances, and data in conclusion.
Discussion and Conclusion: Lab 1A , the data suggests what molecules can and cannot diffuse across a selectively permeable membrane. The coloration showed that the Iodine Potassium
Iodide was small enough to pass through the pores of the membrane because the color of this indicator moved from within the beaker to in the bag. Water and glucose moved out because water is small enough to pass through the membrane and the glucose tested positive with the Testape inside the beaker. The glucose at the beginning was only in the bag, so it obviously moved out.
Lab 1B proved that water moves across the selectively permeable membrane of the dialysis tubing much easier than sucrose sugar does. The water moved to reach equilibrium between the solutions.
Sucrose must be too large a molecule to pass through the membrane quickly.
Lab 1C showed that the potato samples took in water when immersed in a distilled water solution.
Potatoes must contain sucrose molecules due to the conclusion of this lab because the potatoes take in water in the distilled water beaker. Potatoes had a lower water potential and higher solute potential than the distilled water. It is just the opposite inside the beaker.
Lab 1D’s calculations made it evident that all of the results could be determined and proved correct with simple algebra equations and formulas. This gives the lab much better illustration and a stable understanding of diffusion and osmosis.
Lab 1E showed the plasmolysis clearly and allowed the student to see exactly what goes on in this action. This particular part of the lab illustrated the shrinking of the plasma membrane from the cell wall in a plant cell. It shows how plant cells react in a hypertonic environment. The turgor pressure decreases a lot, and the cells become very weak when the water leaves the cell.
BACK