Scientific Notation
advertisement

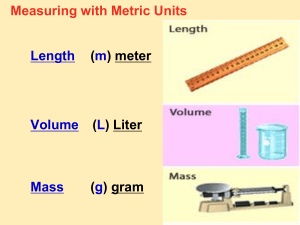
MODULE A - 4 MEASUREMENT SYSTEMS & SCIENTIFIC NOTATION OBJECTIVES • At the end of this module, the student will be able to… Identify and compare the systems of measurement used in the clinical setting. Identify the standard prefixes used in the metric system State the metric units of length, mass, volume, time, and temperature. Distinguish between the metric units for liquid (mL) and solid volume (cc) measurements. Measurement systems • Method of quantifying matter Solids, liquids & gases • Quantities include: Length Area Weight Volume Pressure Temperature Time • Systems used in medicine: A. Conventional B. Metric C. Standard International Conventional Systems • Also known as: British English U.S Customary (FPS) foot, pound, second • Commonly used in U.S. FPS Examples of length & area 12 inches = 1 foot 3 feet (36 inches) = 1 yard 220 yards = 1 furlong 8 furlongs = 1 mile 1,760 yards = 1 mile 5,280 feet = 1 mile 1 sq. foot (foot2) = 122 sq. inches 1 sq. yard (yard2) = 9 sq. feet 43,560 sq. feet = 1 acre 1 sq. mile (mile2) = 640 acres Examples of liquid measure 1 teaspoon (tsp) = 1/ 3 2 tablespoon (tbsp) = 1 fluid ounce 1 fluid ounce (oz) = 1/ 8 cup 2 fluid ounces = 1/ 4 cup 2 2/3 fluid ounces = 1/ 3 cup 4 fluid ounces = 1/ 2 cup 5 1/3 fluid ounces = 2/ 3 cup 6 fluid ounces = 3/ 4 cup 8 fluid ounces = 1 cup 2 cups (c) = 1 pint 2 liquid pints (pt) = 1 liquid quart (qt) 4 liquid pints = 1 gallon (gal) tablespoon Examples of dry measure 1 dry quart = 2 dry pints 8 dry pints = 1 peck 4 pecks = 1 bushel Standard International (SI) • Simplified modification of metric system. • Worldwide effort started in 1960s to standardize to this system. • Also known as: (MKS) meter, kilogram, second MKS Comparison Conventional Units Standard International Units Length inch or foot meter Volume Fluid ounce Liter Cubic Foot (ft3) Area in2 or ft2 m2 Metric System • Developed in Europe. • Has all units based on multiples of 10. • Also known as: (CGS) centimeter, gram, second CGS Measurements in Respiratory Therapy • Length Meter (m) • Volume Liter (L) • Mass Gram (g) • Time Seconds (sec) • Temperature Centigrade (Celsius), Kelvin, Fahrenheit • Pressure Centimeters of Water (cm H2O), Pounds per square inch (psi), Millimeters of mercury (mm Hg), Torr, Pascal (Pa), and Atmospheres (atm) • Force Dynes Conversion • Conversion within the metric system is easy Everything based on multiples of ten. • Conversion from one system to the other: Must know the conversion factors. Conversion • Conversion within these systems or from one system to the other: You Must know how to do metric conversions. I will provide the S.I. and conventional factors on an exam or quiz. • There are too many to memorize. • Gimli Glider & Mars Climate Orbiter Basic (fundamental) Units • Basic unit has value of one. (1x100 = 1) One Liter • Smaller - milliliter • Larger - kiloliter One Gram • Smaller – microgram • Larger - hectogram Larger One Meter • Smaller - decimeter • Larger - Megameter Smaller Opposite of the number line Metric Chart Basic or Fundamental Unit Liter Gram Meter 105 104 103 102 101 100 10-1 10-2 10-3 10-4 10-5 |-------|-------|-------|-------|-------|-------|-------|-------|------|-------| kilo hecto deca x1000 x100 x10 (k) (h) (da) LARGER deci centi milli 1 1 1 10 100 1000 (d) (c) (m) SMALLER Greek Prefixes - Units to the left of the basic unit and larger. • BASIC UNIT = One Liter, Gram or Meter • • • • • • • • • 10 1 10 2 10 3 10 4 10 5 10 6 10 7 10 8 10 9 deca (da) hecto (h) kilo (k) 10 x larger 100 x larger 1000 x larger 10 100 1000 Mega (M) 1,000,000x 1,000,000 Giga (G) 1,000,000,000x 1,000,000,000 Latin Prefixes Units to the right of the basic unit and smaller. • BASIC UNIT = One Liter, Gram or Meter • 10 -1 deci (d); 10 x smaller; 1/10; x 0.1 • 10 -2 centi (c); 100 x smaller; 1/100; x 0.01 • 10 -3 milli (m); 1000 x smaller; 1/1,000; x 0.001 • 10 -4 • 10 -5 • 10 -6 micro (m) or (mc); 1,000,000 x smaller; 1/1,000,000; x 0.000001 • 10 -7 • 10 -8 • 10 -9 nano (n); 1,000,000,000 x smaller; 1/1,000,000,000; x 0.000000001 • 10-10 Angstrom (Å); 10,000,000,000 x smaller; 1/10,000,000,000; x 0.0000000001 Scientific Notation • A method of expressing the value of a very small or very large number. • Scientific Notation: (base exponent) Base is the number to be multiplied by itself (usually 10). Exponent is the number of times it is multiplied. • 103 = 10 x 10 x 10 = 1,000 Scientific Notation Example: • A kilometer is 1,000 times larger than a meter • Count the zeros (that equals exponent) • 103 • 10x10x10 times larger Scientific Notation Example: • Angstrom (Å) is 10 billion times smaller than a meter (m) • That is…10,000,000,000 times smaller • Count the zeros to determine exponent 10 10 or 1 1010 or 1 10,000,000,000 • Can also be written as 0.0000000001 • 10x10x10x10x10x10x10x10x10x10 times smaller Numbers and Exponents 100= 1 a x 100 = a 101= 10 a x 101 = a x 10 102= 100 a x 102 = a x 100 103= 1000 a x 103 = a x 1000 106= 1,000,000 a x 106 = a x 1,000,000 109= 1,000,000,000 a x 109 = a x 1,000,000,000 10-1 = 0.1 a x 10-1 = a x 0.1 10-2 = 0.01 a x 10-2 = a x 0.01 10-3 = 0.001 a x 10-3 = a x 0.001 10-6 = 0.000001 a x 10-6 = a x 0.000001 10-9 = 0.000000001 a x 10-9 = a x 0.000000001 Numbers and Exponents Positive exponent = # of zeros 5 x 100 = 5 5 x 101 = 50 5 x 102 = 500 5 x 103 = 5000 5 x 106 = 5,000,000 5 x 109 = 5,000,000,000 Negative exponent = # of decimal places 5 x 10-1 = 0.5 5 x 10-2 = 0.05 5 x 10-3 = 0.005 5 x 10-6 = 0.000005 5 x 10-9 = 0.000000005 Examples - Avogadro’s Number Expresses the number of atoms in one mole of a gas Long form: 602,000,000,000,000,000,000,000 atoms Scientific notation: 6.02 x 10 23 atoms Process: Count over to the left, the number of decimal places to get a number between 1 & 10 Example - Mass of an electron Long Form: 0.000 000 000 000 000 000 000 000 000 000 911 grams Scientific Notation: 9.11 x 10-31 grams Process: Count over to the right the number of decimal places necessary to get a number between 1 and 10 Practice: Express the following exponentially • 500 = 5 x 102 (count over to left 2 decimal places) • 93,000,000 = _________________ • 0.0003 = _________________ • 0.000000024 = _________________ Exponent Relationship to Basic Unit • Negative exponents are smaller (10 –3) • Positive exponents are larger (10 3) If the metric system was money… | | | | $1,000.00 $100.00 $10.00 $1.00 Basic Unit | | 10 cent 1cent 0.10 0.01 One more point regarding units of measure. Why is mL and cc (cm3) the same? • Cubic centimeter (cc or cm3) and millimeter (mL) are used interchangeably in medicine. The unit cc is a length measurement. The unit mL is a volume measure. • A cube 1 cm long x 1 cm wide by 1 cm high (l x w x h = area) will hold 1 mL of liquid volume. • We therefore use the units interchangeably. 1 cc or cm3 = 1 mL The volume of this cube 1 cm deep Cubic centimeter 1 cm length is one mL. 1 cm high 1 mL = 1 cc = 1 cm3 Additional Conversion Factors Length: 1 meter = 39.37 inches 1 cm = .3937 inches 1 km = 0.62 miles Volume: 1 mL = 1 cc = 1L = 1.0567 qts. 946 mL = 1 qt. 1 pint = 473 mL 1 kg = 2.2 pounds (lbs) 1 lb = 454 grams 1 cm3