Nitrogen cycle
advertisement

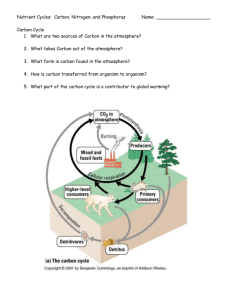
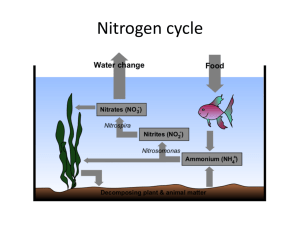
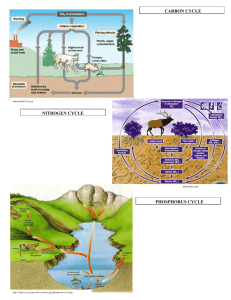
CHOPKIN’S CaFe The primary source of the energy that drives the cycles is the sun The water, nitrogen, phosphorus and carbon cycles are closed systems. Everything is kept within the system, nothing leaves nor enters it So nutrients a not endless but are recycled but in limited amounts Carbon, nitrogen and phosphorus have to be recycled and reused and for this, they are called biogeochemical cycles Nutrients such as nitrogen and phosphorus may be carried away deep ocean and lost from the cycles. Water Cycle Water Cycle Water Cycle Water Cycle Water Cycle Water Cycle Water cycle Earth has a stable water supply with 98% is in abiotic features like oceans and lakes 2% is in ice, water vapor and in living organisms The cycle is driven by the sun Solar heat causes evaporation from bodies of water Solar heat also causes transpiration from trees Evapotranspiration is evaporation from soil and plants Water is drawn into the atmosphere by evaporation and falls back to earth by precipitation. Rain, sleet, hail and snow are four different means of precipitation There is a constant movement of water through the biotic and abiotic reservoirs Runoff may take a long time to reenter the water cycle if it seeps down into the soil and into an underground aquifer,(underground reservoir) Nitrogen cycle NH3 ammonia = NH4 ammonium = NO2 nitrite = NO3 nitrate = N2 Nitrogen is one of the most essential elements on earth. All living things have N in their amino acids. 78% of it is in the atmosphere and is unusable Instead is used in forms of ammonia and nitrates Used in fertilizers that help plants to grow In aquatics, N as a major nutrient for aquatic lifeforms is our concern Can be toxic in high concentrations (ammonia) The cycle begins with nitrogen fixation Nitrogen is fixed chemically by being converted to ammonium NH4 Specialized bacteria only can accomplish this but is a very slow process. Requires an entire season It can be artificially done with CH4, methane Ammonification, nitrification and assimilation Ammonification – caused by water or soil saphrophytes or decomposers Decompose organic compounds and release ammonium to be used by plants and phytoplankton Nitrification – occurs when other nitrogen-fixing bacteria oxidize ammonia or ammonium to produce energy used to make nitrite NO2. This is toxic to fish and plants. Another type of bacteria convert it to nitrate NO3 Assimilation – when organisms utilize ammonium or nitrate within their cells to build protein. Protists and animals die, urinate of defecate, they release their nitrogen compounds back to the earth where saprobes decompose the material to simpler forms Nitrogen cycle Nitrogen cycle Nitrogen cycle Nitrogen cycle Nitrogen cycle – Important steps Stage1 – Entry and Accumulation Ammonia is introduced into the water via tropical fish waste, uneaten food, and decomposition. These will break down into ammonia (NH3). Ammonia is harmful to tropical fish. Stage2 – Nitrification Part 1 Soon, bacteria called nitrosomonas will develop and they will oxidize the ammonia essentially eliminating it. The byproduct of ammonia oxidation is Nitrites. So we no longer have ammonia, but we now have another toxin to deal with - Nitrites. Nitrites are just as toxic to tropical fish as ammonia. Stage 3 – Nitrification Part 2 Bacteria called nitrobacter will develop and they will convert the nitrites into nitrates. Nitrates are not as harmful to tropical fish as ammonia or nitrites, but nitrate is still harmful in large amounts. Stage 4 - Denitrification Denitrifying bacteria can breakdown nitrates into harmless nitrogen gas that escapes through the surface of the water. Nitrogen cycle – Impact on oceans Human activities have a significant effect on nitrogen cycling. Production and use of nitrogen fertilizer, combustion of fossil fuels, and planting crops that fix nitrogen have unbalanced the previously stable relationship between fixation and denitrification. Gaseous industrial pollutants foul the air in many cities and wash out in sufficient amounts to constitute “acid rain” in some parts of the industrialized world. Nitrogen – Regulating in an aquarium For a short period of time, a new aquarium is a toxic cesspool. The water may look clear, but don't be fooled. It's loaded with toxins. Fortunately ,bacteria that are capable of converting wastes to safer by-products begin growing in the tank as soon as fish are added. Unfortunately there aren't enough bacteria to eliminate all the toxins immediately, so for a period of several weeks to a month or more, your fish are at risk. Ways to cycle your tank: 1. Use fish food 2. Use substrate or filter media from an established tank 3. Use a liquid bacteria culture, such as Stress Zyme Carbon Cycle Carbon Cycle Carbon Cycle Carbon Cycle Carbon Cycle Carbon Cycle Found in aquatic environments as carbonate. Causes hard water by combining with calcium Will form as a precipitate in warm shallow seas, making limestone Large amounts of carbon are tied up in rocks formed by decomposition of plants and animals Biotic lifeforms use carbon as an essential element. Plants use CO2 to create glucose, starches & fats Deposits of buried organic compounds Sediments containing trees, skeletons, cell walls of plankton end up at the bottom of lakes, oceans Eventually create coal, natural gas and petroleum deposits Cellular respiration and decomposition of plant tissue release Carbon into the atmosphere and are short cycle processes Volcanic eruptions and human activities such as burning fossil fuels quickly release C back into the atmosphere Carbon – Important steps Stage 1 – Entry and accumulation Carbon dioxide enters the waters of the ocean by simple diffusion. Stage 2 – Uptake - Certain forms of sea life biologically fix bicarbonate with calcium (Ca+2) to produce calcium carbonate (CaCO3). This substance is used to produce shells and other body parts by organisms such as coral, clams, oysters. - Marine plants in the sunlit surface layer of the ocean grab carbon dioxide from the air to use in photosynthesis - When the plants die, they sink as so-called “marine snow” to the deep ocean where the carbon is stored and prevented from re-entering the atmosphere. Stage 3 - At the surface of the oceans where the water becomes warmer, dissolved carbon dioxide is released back into the atmosphere. Carbon – Impact on oceans In the aquatic ecosystem carbon dioxide can be stored in rocks and sediments. It will take a long time before this carbon dioxide will be released, through weathering of rocks or geologic processes that bring sediment to the surface of water. Carbon dioxide that is stored in water will be present as either carbonate or bicarbonate ions. These ions are an important part of natural buffers that prevent the water from becoming too acidic or too basic. When the sun warms up the water carbonate and bicarbonate ions will be returned to the atmosphere as carbon dioxide. Phosphorus Cycle Our atmosphere contains no phosphorus Mainly found as phosphates in the earth’s crust then released into the soil after it rains. Similar to hydrolysis but is really carbonation Plants use phosphates in the soil and animals eat plants recycling the phosphorus Phosphorus is present in fertilizers and can over-enrich aquatic environments and cause algae blooms Bacteria respond and grow consuming oxygen and causing fish kills and eutrophication Phosphorus remains a phosphate mineral deposit in the oceans until any geological uplift exposes it at the surface, (plate tectonics) Eutrophication is an increase in the concentration of phosphorus, nitrogen and other plant nutrients in lakes or oceans Phosphorus Cycle Phosphorus Cycle Phosphorus Cycle Phosphorus Cycle Phosphorus – Impact on oceans Phosphorus is usually present in natural water as phosphates. Phosphorus is a plant nutrient needed for growth and a fundamental element in the metabolic reactions of plants and animals (hence its use in fertilizers). Sources of phosphorus include human and animal wastes (i.e., sewage), industrial wastes, soil erosion, and fertilizers. Excess phosphorus causes extensive algal growth called "blooms," which are a classic symptom of cultural eutrophication and lead to decreased oxygen levels in various bodies of water. Phosphorus – Important Steps Stage 1 – Entry and accumulation Phosphorus is not highly soluble, binding tightly to molecules in soil, therefore it mostly reaches waters by traveling with runoff soil particles Weathering is the breaking down of rocks, soils and minerals through direct contact with the water Leaching is the loss of mineral and organic solutes due to percolation from soil Stage 2 – Uptake Plants dissolve ionized forms of phosphate. Herbivores obtain phosphorus by eating plants, and carnivores by eating herbivores. Herbivores and carnivores excrete phosphorus as a waste product in urine and feces. Stage 3 – Decomposition, Sedimentation, and Uplift Phosphorus is released back to the soil when plants or animal matter decomposes The final resting place for Phosphorus is in the ocean sedimentary beds, where it will eventually return to use via uplifting of sedimentary rock. Phosphorus – Regulating in an aquarium Fortunately phosphates do not directly harm your fish, even at high levels. However, the algae blooms that result from elevated phosphates can ultimately cause problems for the aquarium inhabitants. For instance, green water can deplete the oxygen, which in turn can harm the fish. Techniques to regulating phosphorus levels: 1. Water Change – Large water changes will help bring phosphates down quickly, but if the underlying sources are still there, it will only be temporary 2. Tank Cleaning – Scrape the inside of the glass, remove the rocks and other decorations and scrub them well 3. Phosphate Absorber – Phosphate absorbing media is very effective. It can be added to virtually any filter. NOTE: Generally using chemicals should be your last resort. Water – Important steps The major physical components of the global water cycle include the evaporation from the ocean and land surfaces, the transport of water vapor by the atmosphere, precipitation onto the ocean and land surfaces, the net atmospheric transport of water from land areas to ocean, and the return flow of fresh water from the land back into the ocean. The additional components of oceanic water transport are few, including the mixing of fresh water through the oceanic boundary layer, transport by ocean currents, and sea ice processes Evaporation – Precipitation + Runoff = surface salinity of the ocean Water – Impact on oceans The ocean holds 97% of the total water on the planet. Besides affecting the amount of atmospheric water vapor and hence rainfall, evaporation from the sea surface is important in the movement of heat in the climate system. The ocean is one of Earth's most valuable natural resources. It provides food in the form of fish and shellfish—about 200 billion pounds are caught each year. It's used for transportation—both travel and shipping. It provides a treasured source of recreation for humans. It is mined for minerals and drilled for crude oil. Oxygen Cycle Figure 1. Oxygen dynamics in coastal waters. Processes that increase dissolved oxygen concentrations are shown with green boxes. Processes that decrease dissolved oxygen concentrations are shown with orange boxes. Oxygen – Important steps Stage 1 – Entry -diffusion; oxygen is constantly entering the water from the air, aeration; oxygen is circulated through and dissolved in water, and photosynthesis by plants and algae Stage 2 – Uptake Respiration by animals and degassing – breaking down CO2 and other compounds Stage 3 – Removal oxygen leaves the ocean surface and enters the atmosphere by diffusion Oxygen – Impact on oceans Oxygen in water is known as dissolved oxygen or DO. Adequate dissolved oxygen is necessary for good water quality. Oxygen is a necessary element to all forms of life. Many natural processes require adequate oxygen levels in order to provide for aerobic life forms. Fish and aquatic animals cannot split oxygen from water (H2O) or other oxygen-containing compounds. Only green plants and some bacteria can do that through photosynthesis and similar processes. Virtually all the oxygen we breath is manufactured by green plants. A total of three-fourths of the earth’s oxygen supply is produced by phytoplankton in the oceans.