Nomenclature of Saturated Hydrocarbons
advertisement

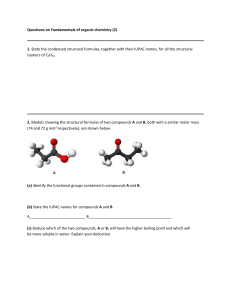
Naming Saturated Hydrocarbons Aliphatic Straight and Branched Alkanes Alkanes At the conclusion of our time together, you should be able to: 1. Define an alkane 2. Name, write and draw various alkanes using the IUPAC naming rules Familiar Sayings The individual of the class aves, arriving before the appointed time, seizes the invertebrate animal of the group vermes. The early bird catches the worm! What are Alkanes? Saturated hydrocarbons Can be straight or with branches The general formula for each alkane is: (CnH2n+2) Materials in Your Kit Make certain your kit contains the following in the right location. If it does not, the previous user will be marked at least 1 point off their next assignment grade each day there is a problem. You are to work with the kit assigned to your lab drawer. 10 black spheres 2 blue spheres 28 yellow spheres 6 red spheres 4 green spheres 2 orange spheres 2 purple spheres - carbon nitrogen hydrogen oxygen chlorine bromine or fluorine iodine \ small side / \ \ \ large side / / (all colors / together) Materials in Your Kit 30 small wooden pegs - H bonds only \ 10 large wooden pegs - other single bonds \ 9 metal springs double or triple bonds / or stress bonds / Place all of these materials in the center section of your kit. Grading The first student lab team that has the structure correct will be initialed by me and receive an extra one-half point. These students will then have their names placed on the board and will be teaching assistants for that organic structure. They will be responsible to grade and initial the structures of other lab teams. Each structure that is designed and named by you is worth 0.5 points. It is best if you put your constructions in the box lid to carry to the instructor or the teaching assistant for that structure. The following is the grading criteria: Grading 1 point = graded by the instructor and perfect, this team assists in grading, may have one 1 point grade and assist with only one structure (RWT with a + ) 0.5 points = other perfect scores (RWT or other initials of student assistants) 0.5 points = (assistants can only give this) 0 points = wrong pegs were used (RWT or other initials of student assistants) 0 points = 1 mistake in the structure or wrong name (RWT or other initials of student assistants) Calvin’s Thoughts on the Season: Some Simple Alkanes (CnH2n+2) 2-methylpropane Cycloalkanes Alkanes Let’s see if you can: 1. Define an alkane 2. Name, write and draw various alkanes using the IUPAC naming rules What are Alkanes? Saturated hydrocarbons Can be straight or with branches The general formula for each alkane is: (CnH2n+2) Let’s Try Naming Some Models? Let’s see if you can build some models yourself?? Grading 1 point = graded by the instructor and perfect, this team assists in grading, may have one 1 point grade and assist with only one structure (RWT with a + ) 0.5 points = other perfect scores (RWT or other initials of student assistants) 0.5 points = (assistants can only give this) 0 points = wrong pegs were used (RWT or other initials of student assistants) 0 points = 1 mistake in the structure or wrong name (RWT or other initials of student assistants) Alkanes At the conclusion of our time together, you should be able to: 1. Define an alkane 2. Name, write and draw various alkanes using the IUPAC naming rules The IUPAC Rules 1. Find the longest carbon chain and name that chain Example: Complete Structure propane C-C-C | C Skeletal Structure CH3-CH-CH3 | CH3 Condensed Structure The IUPAC Rules 1. Find the longest carbon chain and name that chain 2. Number the chain so the substituent groups have the lowest total position numbers 3. Give alkyl groups attached to the longest chain a name and a number Name Side Chains as Alkyl Groups Examples: 2-methylpropane C-C-C | C Complete Structure Skeletal Structure CH3-CH-CH3 | CH3 Condensed Structure A.P. TEST IN MUSIC Write a piano concerto. Orchestrate and perform it with flute and drum. You may use the flute that you received in third grade. The IUPAC Rules 1. Find the longest carbon chain and name that chain 2. Number the chain so the substituent groups have the lowest total position numbers 3. Give alkyl groups attached to the longest chain a name and a number 4. Multiple alkyl groups are named alphabetically 5. Multiple groups that are the same: di(2), tri(3), tetra(4), penta(5), hexa(6) 6. Halogen substituent groups are named “halo” groups – fluoro, chloro, bromo, iodo 7. Put “,” between numbers, “-” between numbers and letters Find The Longest Chain Not pentane!! Number the Longest Chain so the Substituents have the Lowest Numbers Examples: 3-ethylheptane CH3-CH2-CH-CH2-CH2-CH2-CH3 | CH3-CH2 2,7-dimethylnonane CH3-CH-CH2-CH2-CH2-CH2-CH-CH2-CH3 | | CH3 CH3 7th Grade Science Answers "Mushrooms always grow in damp places and so they look like umbrellas." Examples: 4-ethyl-2,4,5-trimethyloctane CH3 CH3 CH3 | | | CH3--CH--CH2---C---CH--CH2--CH2--CH3 | CH3-CH2 Examples: 3,3,4,4-tetraethyl-2,2,5,5-tetramethylhexane CH3 CH3 | | CH3 CH2 CH2 CH3 | | | | CH3-----C-----C-----C-----C-----CH3 | | | | CH3 CH2 CH2 CH3 | | CH3 CH3 Alkanes Let’s see if you can: 1. Define an alkane 2. Name, write and draw various alkanes using the IUPAC naming rules The n-Alkanes CH4 home heating C2H6 alcohol production gas grills C3H8 C4H10 flick your bic C5H12 C6H14 dry cleaners 5-6 C7H16 methane ethane kerosene ~ 12 propane C11H24 undecane butane C12H26 dodecane pentane C13H28 tridecane hexane C14H30 tetradecane heptane C15H32 pentadecane C8H18 gasoline 7-9 octane motor oil ~ 16 C9H20 nonane petroleum jelly 20 C10H22 decane tar ~ 25 wax ~ 40 Give the IUPAC Nomenclature for the Following: All are “pentanes” 2-methylpentane 2,2,4-trimethylpentane 3-ethyl-2-methylpentane Try Naming this One: Another Why - Maxine Why isn't the number 11 pronounced onety-one? The IUPAC Rules 1. Find the longest carbon chain and name that chain 2. Number the chain so the substituent groups have the lowest total position numbers 3. Give alkyl groups attached to the longest chain a name and a number 4. Multiple alkyl groups are named alphabetically 5. Multiple groups that are the same: di(2), tri(3), tetra(4), penta(5), hexa(6) 6. Halogen substituent groups are named “halo” groups – fluoro, chloro, bromo, iodo 7. Put “,” between numbers, “-” between numbers and letters Naming Saturated Hydrocarbons Aliphatic Branched Alkanes Isomers Isomers of Alkanes At the conclusion of our time together, you should be able to: 1. Define an isomer 2. Name, write and draw various isomers of alkanes using the IUPAC naming rules Definition: Isomers are organic compounds that have the same formula but different structure. These structures will have completely different properties. Examples of Isomers of Butane: All Compounds are C4H10 butane CH3-CH2-CH2-CH3 1,1-dimethylethane CH3 | CH3-CH | CH3 2-methylpropane CH3-CH-CH3 | CH3 2-methylpropane Familiar Sayings Compute not your immature gallinaceans prior to their being produced. Don’t count your chickens before they’re hatched!! Examples of Isomers of Pentane: All Compounds are C5H12 pentane CH3-CH2-CH2-CH2-CH3 2,2-dimethylpropane CH3 | CH3-C-CH3 | CH3 2-methylbutane CH3-CH-CH2-CH3 | CH3 What about 3-methylbutane? Bill Gates' Rules Here is a list of 11 things that many high school and college graduates did not learn in school. In his book, Bill Gates talks about how feel-good, politicallycorrect teachings created a full generation of kids with no concept of reality and how this concept has set them up for failure in the real world. RULE 11 Be nice to nerds. Chances are you'll end up working for one. Isomers of Alkanes Let’s see if you can: 1. Define an isomer 2. Name, write and draw various isomers of alkanes using the IUPAC naming rules Give the IUPAC Nomenclature for the Following: All are “pentanes” 2-methylpentane 2,2,4-trimethylpentane 3-ethyl-2-methylpentane