Chemistry
advertisement

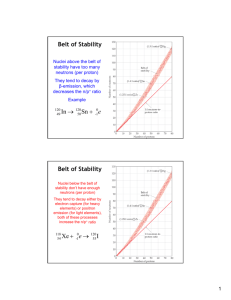
Chemistry MAC NOTES: Nuclear Chemistry Unit • There are four natural forms of radioactive decay for unstable isotopes with gamma rays often released as well but not usually shown in the nuclear equation. • Alpha particle decay is the main way that large unstable isotopes with an atomic number greater than 82 (beyond the band) become more stable. Alpha particles have a mass number of four an atomic number of two and are emitted (released), appearing on the right hand side of the nuclear equation. • Beta particle decay is the only way radioisotopes with a neutron-to-proton ratio above the band of stability become more stable. Beta particles are electrons have no mass number, have a charge of 1- and are emitted (released), appearing on the right hand side of the nuclear equation. • Positron emission is first way that radioisotopes with a neutron–to-proton ratio below the band of stability become more stable. Positron are particles with no mass number, have a charge of 1+ and are emitted (released), appearing on the right hand side of the nuclear equation. • Electron capture is the second way that that radioisotopes with a neutron–to proton ratio below the band of stability become more stable. An electron with no mass number, a 1- charge is drawn into the nucleus, appearing with the parent nuclei on the left hand side of the nuclear equation. 1 Chemistry MAC NOTES: Nuclear Chemistry Unit • The, ‘Band of Stability Graph’ shows about 250 stable isotopes of all naturally occurring elements as a ‘scatter plot’ with the proton number on the X-axis and the number of neutrons on the Y-axis. • There are two reference lines on the graph, a one neutron to one proton line below the band and a 1.5 neutron to one proton line above the band. • Isotope can be between the two reference lines but NOT in the band and so would be considered unstable. • The general trend is for small elements (atomic numbers 1-20) to be stable with a ratio of one neutron to one proton; the number of neutrons required for stability increases as the atomic number increases so that a ratio of 1.5 neutrons to one proton is usually required for larger elements with an atomic number greater than 70. 2 Chemistry MAC NOTES: Nuclear Chemistry Unit • The half-life equation has five parts—N = the amount remaining with a mass unit; No = the original amount with a mass unit; (1/2) = a constant; t = total elapsed time with a time unit; T = time for one half life with a time unit. • Half life problems may ask you to solve for N (an actual amount or as a percent), No, t, or T. Solving for total elapsed time (t) or half life (T) requires the use of the LOG function on your calculator. • Mass defect and binding energy calculations require a four-step calculation process: 1. 2. 3. 4. Determine the mass defect in amu using the total number neutrons, protons, their respective masses, and the mass of the isotope’s nucleus. Convert the mass defect from amu (step 1) to kg using a provided conversion factor. Calculate total binding energy in Joules (J) by multiplying the mass defect in kg (step 2) by the speed of light squared. Calculate the binding energy per nucleon by dividing the total binding energy (step 3) by the number of nucleons. • As mass defect and binding energy per nucleon goes up, the greater the stability of the nucleus and vice versa. 3 Chemistry MAC NOTES: Nuclear Chemistry Unit • Nuclear equations are a short-hand way to show the natural or man-made change in a parent nuclei on the left hand side of the arrow into daughter nuclei on the right hand side of the arrow. • Natural forms of decay will be shown in a nuclear equation with symbols for alpha particles, beta particles, positrons, and electrons being captures. • Man-made fission or fusion will be shown in a nuclear equation with symbols for ‘particle bullets’ such as alpha particles and/or neutrons on the left hand side and protons and neutrons on the right hand side of the equation. • Symbol notation is used for equations that show the mass number above and the charge below the symbol for the isotope or particle. • Nuclear equations demonstrate a conservation of the mass number on the left and right hand side of the equation as well as the number of positive charges (proton count). This can be used to write the correct symbol notation for missing parts of a nuclear equation. • If parent nuclei are larger (larger mass number and atomic number) than the daughter nuclei, then the equation shows fission. • If the parent nuclei are smaller (smaller mass number and atomic number) than the daughter nuclei, then the equation shows fusion. 4