Ch 20 Physical Science Notes Chemical Bonds Marlin Name
advertisement

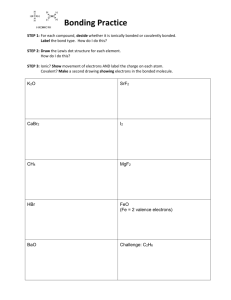
Ch 20 Physical Science Notes Chemical Bonds Marlin Name _____________________________________ Section 1: Stability in Bonds Not all elements bond. Some are uncombined. _____________________ are formed by chemical changes. _________________________ are different once chemical bond compared to when they are unbounded or uncombined. Remember: Na and Cl gas: Na is a metal and Cl2 is poisonous gas. But when combined it is safe to consume. A ________________________________ tell us descriptions of chemical bonds. 2 things chemical formulas tell us: 1. 2. How does the formula describe water (H2O) or carbon dioxide (CO2)? How are elements held together in bonds? _________________________ _________________________________________________________ Which gases are unusually stable and will not bond? __________________ Electron dot diagrams help us see the stability of bonds. Think back to how we look at compounds filling the octet in the valence shell. Just for review list the valence pattern for the periodic table. Page 1 of 12 Consider the example of the bonding of AlBr3. Why doesn’t H and He complete the octet as normal? _________________ _________________________________________________________ As elements come together and complete their octets, they become chemically stable. THIS IS THE REASON ELEMENTS BOND! Remember: the gaining, loss, or sharing of electrons creates the attractive force between elements that is like the glue holding the bond together. The ____________________________________ is the force of attraction of the elements that holds them together. Section 2: Types of Bonds Atoms will either lose or gain to achieve chemical stability. Once the electron is transferred the atom becomes an ____________. Metals ___________ electrons to bond. Nonmetals ____________ electrons to bond. Once this transfer occurs the atom is no longer a neutral charge. Metals are ___________ charged. Nonmetals are ____________ charged. When elements have a charge they can either be attracted or repelled just like MAGNETS. When you think of IONIC BONDING, think MAGNETS. Consider the charges: metals ( ___) and nonmetals (___) will _____________________. Page 2 of 12 Consider the bonding of KI. Start by looking at the electron dot diagram. Notice that when nonmetals are in a bond their name changes. The ending goes from and –ine to an –ide. This will show if a nonmetal is bonded or not bonded. Iodine: NOT bonded (look at ending) Iodide: BONDED (look at ending) _______________________________ is the bonding created by the attractive forces created by the transfer of electrons. During ionic bonding the charged ions work to become neutral as a group. Look at the electron dot diagram of the compound MgCl2. Once this elements have become neutral as a compound, this is called the net zero charge. This method of bonding ions so that the net zero charge is achieved can be seen by the “criss-cross method.” Let’s look at AlBr3: 1. Start with the elements written with the charges. Page 3 of 12 2. Criss-cross the numbers only from superscripts down to subscripts. 3. Check to see is the overall charge of the compound is zero. (net zero charge) Bonds between 2 nonmetals will not have a transfer of electrons. These atoms become chemically stable by sharing electrons. _________________________ is a bond where the force of attraction is created by electrons being shared. The particle that is created from covalent compounds is called a __________________. Covalent bonds can forms different bonds: single, double, and triple bonds. Each bond is determined by how many electrons are shared. Often times the electrons are not shared equally. The only time that we see electrons being shared equally is during diatomic molecule bonding. What are diatomic molecules? _______________________________ Memory tool: _____________________ Unequal sharing occurs when one atom wants electrons more than the other. Atoms that have a high need for electrons are in the top-right hand corner of the periodic table. F has the highest need for electrons compared to ALL other elements. This is how the force of attraction is created for the covalent bonds. Look at the example of HCl. Page 4 of 12 Cl has a stronger attraction for electrons so the electrons move closer to Cl than H. This creates a partial charge. Partial charges are only seen in covalent bonds. Think back to the bondingelectrons are not moving from one element to another. The electrons are shared. When electrons are closer to one element compared to another, this creates unequal sharing or a dipole. Notice the symbol for the dipole, δ. This will be used to show partial charges. When we have molecules that have dipoles, this are called polar molecules. A ________________ ____________________ is a molecule that has one end that is slightly (+) and one end that is slightly (-). The electrons are not equally shared. Other covalently bonded molecules have equally shared electron distribution. These are the opposite of the polar molecules and are called nonpolar molecules. Page 5 of 12 ______________ ________________ are bonds where electrons are distributed equally. Nonpolar molecules are made up of atoms that are identical. Example are the diatomic molecules. Section 3: Writing Formulas and Naming Compounds A compound that is composed of two elements is called a binary compound. In order to know how to write the formula we will need the: 1) Element symbol 2) Charge or oxidation number 3) Net zero charge When getting the oxidation or charge of an element all we need to know is the element’s location on the periodic table. For ionic bonds, the oxidation number is the same as the group charge. For example: group 1 elements will have a charge of +1 and an oxidation of +1. Often times we find transition metals may have more than one oxidation number. This is the reason we did not include them in the charge by group pattern. The net zero charge is expressed by allowing the elements to come together so that all charges from the individual elements balance out. This was seen with our “criss-cross method.” Rules for writing formulas: (follow these steps in order) 1) Write the symbol for the element with the (+) charge. 2) Write the symbol for the element with the (-) charge. Page 6 of 12 3) The charges then need to be balanced out, to the net zero charge. (This is the criss-cross method.) Use subscripts to show the number of each ion used. These will be in the simplest form. REMEMBER: THE NUMBER IS THE ONLY THING TRANSFERRED DURING THE CRISS-CROSS METHOD, NOT THE (+) OR (–) SIGN. Practice: Write the formulas for A. sodium fluoride B. aluminum sulfide C. calcium oxide Rules for Writing Names of Formulas: (follow these steps in order) 1) Write the name of the cation. 2) Add a roman numeral in parenthesis for transition metals. 3) Write the root name of the anion. Add an –ide to the ending of the root name. For naming, it can also be seen as the reverse of the criss-cross method. The cation will always be listed first in the formula and the anion second. This allows you to reverse the subscripts for the numerical values of the charges. For example: AlBr3 1) Name of cation: _____________________ 2) Is it a transition metal? _______________ Page 7 of 12 3) Name of anion: __________________ 4) Reverse the subscripts back to get charge of cation. Al1 Br3 Al+3 Br-1 Name: ________________________________ Fe2O3 1) Name the cation: _____________________ 2) Is it a transition metal? ________________ 3) Name of anion: _______________________ 4) Reverse the subscripts to get the charge of the cation. Name: ________________________________ Some compounds contain more than two elements and are not binary. These compounds can contain polyatomic ions. These are a group of elements covalently bonded together that carry a charge as a group. Page 8 of 12 Notice that none of these are single elements. These polyatomic ions have specific names that will not be changed during the naming process. For example to name CaSO4: Calcium Sulfate Rules for writing formulas with complex ions: Page 9 of 12 Notice the use of parenthesis for polyatomic ions that contain subscripts. Another group of complex ions is named hydrates. Just as the name suggests these compounds have water attached to them. What is the formula for water? __________________ There is a common form for writing the formulas for hydrates. The water is chemically bonded to the compound, but written at the end of the formula. CaSO4·2H2O When writing covalently bonded compounds we will use prefixes to show the number of atoms. Prefixes used: These have to be used because charges are not present in covalent compounds. Rules for Naming Covalent Binary Compounds: (follow these steps in order) 1) List the element with the smallest group number first. Use the name just as on the periodic table. Page 10 of 12 2) Place appropriate subscript to show quantity. 3) List the element with the highest group number next. Replace the ending with –ide. 4) Place appropriate subscript to show quantity. Example: carbon dioxide: 1) Write symbol for carbon: C 2) No prefix so no subscript 3) Write symbol for oxide: O 4) Use subscript that represents “di-“: 2 Formula: CO2 Rules for Writing Formula of Covalent Compound: 1) Write name of the first element. 2) Place prefix in front of the name if necessary. 3) Write the name of the second element. Replace the ending with the –ide. 4) Place prefix in front of name if necessary. Write formula for CCl4: 1) Name of C: carbon 2) Name of Cl: chlorine chloride 3) Use prefix for 4: tetraName : carbon tetrachloride. Page 11 of 12 Naming practice: A. Sodium phosphate decahydrate B. Nitrogen trioxide C. Dihyrogen sulfide Page 12 of 12