CHEN 623-Chemical Engineering Thermodynamics
advertisement

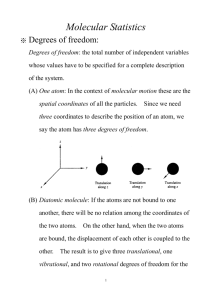
CHEN 623-Chemical Engineering Thermodynamics The “internal” energy: Energies of atoms and molecules Fall 2013 Instructor: Dr. Perla B. Balbuena JEB 240 balbuena@tamu.edu http://research.che.tamu.edu/groups/Balbuena/courses.htm Thermodynamics • Equilibrium • Developed in the 1800’s as experimental science (classical thermodynamics) • Applications to many fields • In the 1900’s statistical thermodynamics • Statistical thermodynamics uses a few concepts of quantum mechanics • Our course focuses on “molecular thermodynamics” 2 Today we are going to talk about contributions to the “internal” energy 3 Electronic energy of the H atom Energy levels are quantized: Ground state, 1st excited state, etc What happens when n is very large? What happens when an electron makes a transition from one state to another? Electromagnetic radiation consists of packets of energy called photons, their energy is hn 4 Emission or absorption of electromagnetic radiation e2 > e 1 energy is absorbed e2 = e1 +h n1-2 how about the energy for the transition 2-> 1? hn = D e D e is the absolute energy difference (Bohr frequency condition) 5 Emission spectrum hydrogen atom 1 1 hn n2 e n e 2 2.17869 x10 18 J 2 2 2 n c=ln 6 The Schrödinger equation for a single particle in one dimension potential energy allowed energy d ( x) V ( x) ( x) e ( x) 2 2m dx 2 2 wave function mass position Solving this eigenvalue equation for a particle in a given potential, we obtain wave eigenfunctions and corresponding allowed energies For the H atom, the allowed energies have the form found experimentally and the wave functions are the hydrogen atomic orbitals 1s, 2s, 2p, etc… 7 what is the wave function? ( x)dx 2 probability to find the particle between x and x+dx specifies the state of the particle for electrons, atoms, molecules, this probability is less sharp than in everyday objects each wave function has an associated energy (en) more than one wave function may be associated with the same energy (degeneracy of the level, gn) 8 Translational Energy So far we discussed only electronic energies Other types of energy: kinetic (translational) Example: a particle constrained to 0 < x < a 2 2 nh en 8ma 2 en n n x y z Allowed translational energies, n =1, 2, 3, … levels are non-degenerate 2 2 2 n h nx nz y 2 2 2 8m a b c 2 Particle in a 3-dimensional volume 9 Electronic energies of atoms other than H • Can be calculated by numerical solutions of the Schrodinger equation • Can be measured (Moore Tables, Atomic Energy Levels, see Tables 1.2, 1.3) 10 First few states of atomic sodium 11 Vibrational energies Let’s consider the diatomic molecule f k ( R Rc ) kx Two masses connected by a spring Hooke’s law k is the force constant if the spring is extended or compressed and then let it undergo its natural motion x(t ) A cos 2nt 1 n 2 1/ 2 k classical harmonic oscillator A: amplitude of the motion : reduced mass 12 Internuclear potential of a diatomic molecule harmonic potential is a good approximation at small displacements 13 Energy levels of a quantum mechanical harmonic oscillator QM solution of the Schrodinger equation for the one-dimensional Harmonic oscillator : h ev 2 1/ 2 k 1 1 v hn v 2 2 v = 0, 1, 2,… levels are non-degenerate The first level (v =0) is not zero zero point energy 14 Infrared (IR) spectrum of a diatomic molecule a transition for one vibrational state to another one : absorbing or emitting EM radiation De hn observed only transitions between adjacent states are allowed Dn = h De en 1 en 2 +1 1/ 2 k hn c=ln 1/ 2 n~ obs 1 k 2c observed frequency in wavenumbers, cm-1 fundamental vibrational frequency for diatomic molecules, these frequencies appear in the IR region 15 IR spectrum of HCl 16 Dissociation energy (Do)and ground state electronic energy (De) Anharmonic oscillator: HCl hn Do De 2 17 Rigid rotator distance between the masses is fixed (Re) note: an approximation since there is also vibration CoM given by m1R1 = m2R2 The molecule rotates around the CoM at a frequency nrot; define angular velocity w 2nrot > u1 2R1nrot R1w; u2 2R2nrot R2w Kinetic energy of the rigid rotator K 1 1 1 1 2 2 2 2 m1n 1 m2n 2 (m1 R1 m2 R2 )w 2 Iw 2 2 2 2 2 I is the moment of inertia 18 Solving the Schrödinger equation for a rigid rotator Allowed energies: Degeneracy: 2 e J J ( J 1) 2I J = 0, 1, 2, … g J 2J 1 Transitions are allowed only for adjacent states, so DJ = +1 De n h2 4 I 2 h 4 I 2 ( J 1) J = 0, 1, 2, … ( J 1) 19 Microwave spectroscopy n h 4 I 2 ( J 1) 2 B ( J 1) B is the rotational constant of the molecule 20 Vibrations of polyatomic molecules A molecule has n nuclei; complete specification requires 3n coordinates (3n degrees of freedom): 3 for the center of mass (translational degrees of freedom) For a linear molecule, 2 coordinates needed to define orientation about its C of M (2 degrees of rotational freedom); remaining coordinates: 3n-5 specify the relative positions of the n nuclei (vibrational degrees of freedom). For a nonlinear molecule, 3 degrees of rotational freedom needed; remaining coordinates: 3n-6 specify the relative positions of the n nuclei (vibrational degrees of freedom). 21 22 23 24 Normal modes CO2 25 Molecules that are IR active • Their dipole moment changes during the motion of the normal mode (condition to be IR active) • What happens with CO2? What modes are active? 26 27 Rotational spectrum of a polyatomic molecule • Same energy levels as in the diatomic, but the moment of inertia depends on the configuration of the molecule (see section 1.10) 28 The energy of a molecule in the rigid-rotator harmonic-oscillator approximation • Atom: translational + electronic • Diatomic Molecule: translational +rotational + vibrational + electronic • Polyatomic Molecule: translational +rotational + vibrational + electronic. However rotational contribution depends on I, and vibrational sums over all modes: 1 nvib e vib hn j (u j ) j 1 2 u j 0,1,2... 29 1st homework-Due Friday, Sept. 6th • Problems 1.24; 1.41; 1.42; 1.44; 1.46; 3.1; 3.5; 3.6; 3.9; 3.10 • For practice try other problems as well, although they won’t be graded 30