SOL Review
advertisement

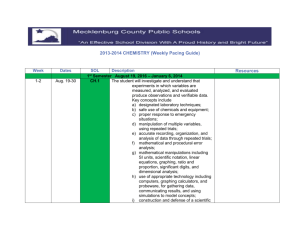
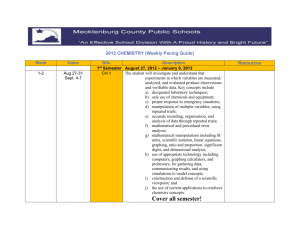
BIGGER PICTURE LAST UNIT/Experience Solutions and Acids/Bases UNIT SCHEDULE Date DO/ Due 4/24 (A) & 4/23 (B) NOTES & DEMO LAB/ Equilibrium Practice 4/28 (A) & 4/27 (B) NOTES & ACTIVITY/ 4/30 (A) & 4/29 (B) TEST & ANALYSIS/ Chemistry SOL Review Scientific Investigation Jlab Project Due by 4pm 5/6(A) & 5/5 (B) CH 1 & CH 3 5/8 (A) & 5/7 (B) CH 2 & CH 5 5/12 (A) & 5/11(B) SOL Review 5/14 CHEMISTRY SOL Atomic Structure and Periodic Relationships Learning Targets 1. 2. 3. 4. 5. 6. is about Study of Matter Pretest ACTIVITY CH 4/ Memorization Test (50 pts) Labs UNIT MAP Lab- Equilibrium and LeChatelier’s Principle 5/4(A) & 5/1 (B) NEXT UNIT/Experience CURRENT UNIT The student will investigate and understand that experiments in which variables are measured, analyzed, and evaluated produce observations and verifiable data. The student will investigate and understand that the placement of elements on the periodic table is a function of their atomic structure The student will investigate and understand how basic chemical properties relate to organic chemistry and biochemistry The student will investigate and understand how conservation of energy and matter is expressed in chemical formulas and balanced equations. The student will investigate and understand that chemical quantities are based on molar relationships. The student will investigate and understand that the phases of matter are explained by kinetic theory and forces of attraction between particles. Chemical Formulas and Reactions Phases of Matter and Kinetic Molecular Theory Molar Relationships UNIT VOCABULARY Rank your understanding of the vocabulary in this unit: (3) I know it!, (2) I'm close to knowing it, (1) I have no idea __ pressure __ stoichiometric relationships __ temperature __ solution concentrations __ volume __ partial pressure __ acid/base theory __ gas laws __ strong electrolytes __ vapor pressure __ weak electrolytes __ phase changes __ nonelectrolytes __ molar heat of fusion __ molar heat of vaporization __ dissociation __ ionization __ specific heat capacity __ colligative properties __ pH and pOH __ Avogadro’s principle __ titration __ molar volume __ organic chemistry __ biochemistry __ nomenclature __ balancing chemical equations __ writing chemical formulas __ bonding types __ reaction types __ reaction rates __ kinetics __ equilibrium __ SI units __ scientific notation __ significant digits __ dimensional analysis