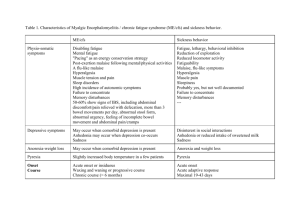
Effects of Acute Inflammation
advertisement

Acute Inflammation
Introduction
Inflammation is the response of living tissue to damage.
The acute inflammatory response has 3 main functions.
1.The affected area is occupied by a transient material called the acute inflammatory exudate. The
exudate carries proteins, fluid and cells from local blood vessels into the damaged area to mediate local
defenses.
2.If an infective causitive agent (e.g. bacteria) is present in the damaged area, it can be destroyed and
eliminated by components of the exudate.
3.The damaged tissue can be broken down and partialy liquefied, and the debris removed from the site
of damage.
The cause of acute inflammation may be due to physical damage, chemical substances, micro-organisms or
other agents. The inflammatory response consist of changes in blood flow, increased permeability of blood
vessels and escape of cells from the blood into the tissues. The changes are essentially the same whatever the
cause and wherever the site.
Acute inflammation is short-lasting, lasting only a few days. If it is longer lasting however, then it is referred
to as chronic inflammation. Various examples of acute inflammation that you may be aware of are sore
throat, reactions in the skin to a scratch or a burn or insect bite, and acute hepatitis and so on. However, there
are occasional historical exceptions such as pneumonia, inflammation of the lung rather than pneumonitis
and pleurisy, inflammation of the pleura, rather than pleuritis.
Clinical Aspects of Acute Inflammation
The four principal effects of acute inflammation were described nearly 2,000 years ago by Celcus:
Redness (rubor) ROSSORE
An acutely inflamed tissue appears red, for example skin affected by sunburn, cellulitis due to bacterial infection or acute
conjunctivitis. This is due to dilatation of small blood vessels within the damaged area.
Heat (calor) CALORE
Increase in temperature is seen only in peripheral parts of the body, such as the skin. It is due to increased blood flow
(hyperaemia) through the region, resulting in vascular dilatation and the delivery of warm blood to the area. Systemic fever,
which results from some of the chemical mediators of inflammation, also contributes to the local temperature.
RIGONFIAMENTO
Swelling (tumor)
Swelling results from oedema, the accumulation of fluid in the extra vascular space as part of the fluid exudate, and to a
much lesser extent, from the physical mass of the inflammatory cells migrating into the area.
DOLORE
Pain (dolor)
For the patient, pain is one of the best known features of acute inflammation. It results partly from the stretching and
distortion of tissues due to inflammatory oedema and, in particular, from pus under pressure in an abscess cavity. Some of the
chemical mediators of acute inflammation, including bradykinin, the prostaglandins and serotonin, are known to induce pain.
Loss of function ALTERAZIONE DI FUNZIONE
Loss of function, a well-known consequence of inflammation, was added by Virchow (1821-1902) to the list of features
drawn up by Celsus. Movement of an inflamed area is consciously and reflexly inhibited by pain, while severe swelling may
physically immobilise the tissues.
Causes of Acute Inflammation
Microbial infections
One of the commonest causes of inflammation is microbial infection. Viruses lead to death of individual cells by
intracellular multiplication . Bacteria release specific exotoxins - chemicals synthesised by them which specifically
initiate inflammationÑor endotoxins, which are associated with their cell walls. Additionally, some organisms cause
immunologically-mediated inflammation through hypersensitivity reactions. Parasitic infections and tuberculous
inflammation are instances where hypersensitivity is important.
Hypersensitivity reactions
A hypersensitivity reaction occurs when an altered state of immunological responsiveness causes an inappropriate or
excessive immune reaction which damages the tissues. The types of reaction are classified here, but all have cellular or
chemical mediators similar to those involved in inflammation.
Physical agents
Tissue damage leading to inflammation may occur through physical trauma, ultraviolet or other ionising radiation, burns
or excessive cooling ('frostbite').
Irritant and corrosive chemicals
Corrosive chemicals (acids, alkalis, oxidising agents) provoke inflammation through gross tissue damage. However,
infecting agents may release specific chemical irritants which lead directly to inflammation.
Tissue necrosis
Death of tissues from lack of oxygen or nutrients resulting from inadequate blood flow (infarction) is a potent
inflammatory stimulus. The edge of a recent infarct often shows an acute inflammatory response.
COMPONENTI CELLULARI E NON CELLULARI CHE PARTECIPANO
ALLO SVILUPPO DEL PROCESSO INFIAMMATORIO
Figure 2-1 The components of acute and chronic inflammatory responses: circulating cells and proteins, cells of blood vessels, and cells and proteins of the extracellular
matrix.
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
MEDIATORI DELL’INFIAMMAZIONE
Figure 2-12 Chemical mediators of inflammation. EC, endothelial cells.
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
Figura 13.34 - Effetti locali e sistemici delle citochine infiammatorie.
Dal volume: Pontieri “Patologia Generale”
Piccin Nuova Libraria S.p.A.
Early Stages of Acute Inflammation
In the early stages, oedema fluid, fibrin and neutrophil polymorphs accumulate in the extracellular spaces of
the damaged tissue. The presence of the cellular component, the neutrophil polymorph, is essential for a
histological diagnosis of acute inflammation. The acute inflammatory response involves three processes:
•changes in vessel calibre and, consequently, flow
•increased vascular permeability and formation of the fluid exudate
•formation of the cellular exudate by emigration of the neutrophil polymorphs into the extravascular
space.
Briefly, the steps involved in e acute inflammatory response are:
1.Small blood vessels adjacent to the area of tissue damage initially become dilated with increased
blood flow, then flow along them slows down.
2.Endothelial cells swell and partially retract so that they no longer form a completely intact internal
lining.
3.The vessels become leaky, permitting the passage of water, salts, and some small proteins from the
plasma into the damaged area (exudation). One of the main proteins to leak out is the small soluble
molecule, fibrinogen.
4.Circulatirlg neutrophil polymorphs initially adhere to the swollen endothelial cells (margination), then
actively migrate through the vessel basement membrane (emigration), passing into the area of tissue
damage.
5.Later, small numbers of blood monocytes (macrophages) migrate in a similar way, as do Iymphocytes.
Figure 2-2 The major local manifestations of acute inflammation, compared to normal. (1) Vascular dilation and increased blood flow (causing erythema and warmth), (2)
extravasation and deposition of plasma fluid and proteins (edema), and (3) leukocyte emigration and accumulation in the site of injury.
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
Figure 2-3 Blood pressure and plasma colloid osmotic forces in normal and inflamed microcirculation. A, Normal hydrostatic pressure (red arrows) is about 32 mm Hg at
the arterial end of a capillary bed and 12 mm Hg at the venous end; the mean colloid osmotic pressure of tissues is approximately 25 mm Hg (green arrows), which is
equal to the mean capillary pressure. Although fluid tends to leave the precapillary arteriole, it is returned in equal amounts via the postcapillary venule, so that the net
flow (black arrows) in or out is zero. B, Acute inflammation. Arteriole pressure is increased to 50 mm Hg, the mean capillary pressure is increased because of arteriolar
dilation, and the venous pressure increases to approximately 30 mm Hg. At the same time, osmotic pressure is reduced (averaging 20 mm Hg) because of protein
leakage across the venule. The net result is an excess of extravasated fluid.
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
Figure 2-3 Blood pressure and plasma colloid osmotic forces in normal and inflamed microcirculation. A, Normal hydrostatic pressure (red arrows) is about 32 mm Hg at
the arterial end of a capillary bed and 12 mm Hg at the venous end; the mean colloid osmotic pressure of tissues is approximately 25 mm Hg (green arrows), which is
equal to the mean capillary pressure. Although fluid tends to leave the precapillary arteriole, it is returned in equal amounts via the postcapillary venule, so that the net
flow (black arrows) in or out is zero. B, Acute inflammation. Arteriole pressure is increased to 50 mm Hg, the mean capillary pressure is increased because of arteriolar
dilation, and the venous pressure increases to approximately 30 mm Hg. At the same time, osmotic pressure is reduced (averaging 20 mm Hg) because of protein
leakage across the venule. The net result is an excess of extravasated fluid.
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
Figure 2-4 Diagrammatic representation of five mechanisms of increased vascular permeability in inflammation (see text).
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
Fattori condizionanti l’accumulo di fluido in un tessuto
P = pressione idrostatica capillare ( Pc ) o interstiziale ( Pif )
P = pressione “oncotica” capillare ( P pl ) o interstiziale ( Pif )
(Pc – Pif) - (P pl – Pif)
capillare arterioso
capillare venoso
Pc
25.0 mmHg
10.0 mmHg
Pif
- 6.3
- 6.3
P pl
28.0
28.0
Pif
5.0
5.0
+ 8.3
- 6.7
PATOGENESI EDEMI TRASUDATIZI
GENERALIZZATO
LOCALIZZATO
Trombosi venosa
Ridotto drenaggio linfatico
Ipervolemia: aumentato riassorbimento
Acqua per riduzione della P arteriosa
(insufficienza cardiaca)
AUMENTO DELLA PRESSIONE IDROSTATICA CAPILLARE VENOSA
DIMINUZIONE DELLA PRESSIONE ONCOTICA
Diminuita sintesi (patologia epatica,
Denutrizione) o aumentata perdita
(alterazioni filtrazione glomerulare)
di proteine
Effects of Acute Inflammation
The systemic effects of acute inflammation have been discussed previously. The local effects are usually clearly
beneficial, for example the destruction of invading microorganisms; but at other times they appear to serve no
obvious function, or may even be positively harmful.
Beneficial effects
Both the fluid and cellular exudates may have useful effects. Beneficial effects of the fluid exudate are as follows:
Dilution of toxins. Dilution of toxins, such as those produced by bacteria, allows them to be carried away in Iymphatics.
|
Entry of antibodies. Increased vascular permeability allows antibodies to enter the extravascular space, where they may lead either
to Iysis of microorganisms, through the participation of complement, or to their phagocytosis by opsonisation. Antibodies are also
important in neutralisation of toxins.
Drug transport. The fluid carries with it therapeutic drugs such as antibiotics to the site where bacteria are multiplying.
Fibrin formation. Fibrin formation from exuded fibrinogen may impede the movement of micro-organisms, trapping them and so
facilitating phagocytosis.
Delivery of nutrients and oxygen. Delivery of nutrients and oxygen, essential for cells such as neutrophils which have high
metabolic activity, is aided by increased fluid flow through the area.
Stimulation of immune response. The drainage of this fluid exudate into the Iymphatics allows particulate and soluble antigens to
reach the local Iymph nodes where they may stimulate the immune response.
The role of neutrophils in the cellular exudate is described here. They have a life-span of only 13 days and must be constantly
replaced. Most die locally, but some leave the site via the Iymphatics. Blood monocytes also arrive at the site and, on leaving the
blood vessels, transform into macrophages, becoming more metabolically active, motile and phagocytic. Phagocytosis of microorganisms is enhanced by opsonisation by antibodies or by complement. In most acute inflammatory reactions, macrophages play a
lesser role in phagocytosis compared with that of neutrophil polymorphs. They appear late in the response and are usually
responsible for clearing away tissue debris and damaged cells. Both neutrophils and macrophages may discharge their Iysosomal
enzymes into the extracellular fluid by exocytosis, or the entire cell contents may be released when the cells die. Release of these
enzymes assists in the digestion of the inflammatory exudate.
Effects of Acute Inflammation
Harmful effects
The release of Iysosomal enzymes by inflammatory cells may also have harmful effects:
Digestion of normal tissues. Enzymes such as collagenases and proteases may digest normal
tissues, resulting in their destruction. This may result particularly in vascular damage, for
example in type III hypersensitivity reactions and in some types of glomerulonephritis.
Swelling. The swelling of acutely inflamed tissues may be harmful: for example, the swelling of
the epiglottis in acute epiglottitis in children due to Haemophilus influenzae infection may
obstruct the airway, resulting in death. Inflammatory swelling is especially serious when it
occurs in an enclosed space such as the cranial cavity. Thus, acute meningitis or a cerebral
abscess may raise intracranial pressure to the point where blood flow into the brain is impaired,
resulting is ischaemic damage, or may force the cerebral hemispheres against the tentorial
orifice and the cerebellum into the foramen magnum (pressure coning).
Inappropriate inflammatory response. Sometimes, acute inflammatory responses appear
inappropriate, such as those which occur in type I hypersensitivity reactions (e.g. hay fever)
where the provoking environmental antigen (e.g. pollen) otherwise poses no threat to the
individual. Such allergic inflammatory responses may be life-threatening, for example extrinsic
asthma.
Figure 2-8 Schematic and histologic sequence of events following acute injury. The photomicrographs are representative of the early (neutrophilic) (left) and later
(mononuclear) cellular infiltrates (right) of infarcted myocardium. The kinetics of edema and cellular infiltration are approximations. For sake of simplicity, edema is shown
as an acute transient response, although secondary waves of delayed edema and neutrophil infiltration can also occur.
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
Figure 2-6 The multistep process of leukocyte migration through blood vessels, shown here for neutrophils. The leukocytes first roll, then become activated and adhere to
endothelium, then transmigrate across the endothelium, pierce the basement membrane, and migrate toward chemoattractants emanating from the source of injury.
Different molecules play predominant roles in different steps of this process-selectins in rolling; chemokines in activating the neutrophils to increase avidity of integrins (in
green); integrins in firm adhesion; and CD31 (PECAM-1) in transmigration.
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
Figure 2-6 The multistep process of leukocyte migration through blood vessels, shown here for neutrophils. The leukocytes first roll, then become activated and adhere to
endothelium, then transmigrate across the endothelium, pierce the basement membrane, and migrate toward chemoattractants emanating from the source of injury.
Different molecules play predominant roles in different steps of this process-selectins in rolling; chemokines in activating the neutrophils to increase avidity of integrins (in
green); integrins in firm adhesion; and CD31 (PECAM-1) in transmigration.
Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 21 October 2005 01:12 PM)
© 2005 Elsevier
Figura 13.31 - Patogenesi della risposta infiammatoria acuta e cronica.
Dal volume: Pontieri “Patologia Generale”
Piccin Nuova Libraria S.p.A.