New Cell Resp
advertisement

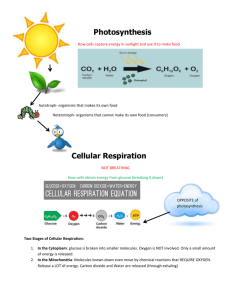
Cellular Respiration Harvesting Chemical Energy First Law of Thermodynamics • Energy is the ability to bring about change or do work. • The first law of thermodynamics states that: energy can be changed from one form to another, but it cannot be created or destroyed. • For cells this means that the energy from ATP must be obtained from another energy source such as the organic molecules derived from photosynthesis. And the energy to create molecules such as glucose must come from another energy source (ie-light). Second Law of Thermodynamics • In the process of energy transfer, some energy will dissipate as heat. • For cells this means that during either energy use or storage there will inevitably be some energy lost as heat. These processes are inefficient. Cellular Respiration • Cellular respiration is the process by which organic molecules (ie-glucose) in combination with oxygen are converted into usable energy (ie-ATP), carbon dioxide and water. • Energy storage in the biosphere can be viewed as a balance between photosynthetic and cellular respiratory activities. Metabolism • Metabolism: the sum of all chemical reactions that occur in the body (ATP made vs. ATP used) • There are two types of metabolism » anabolism » catabolism Photosynthesis as an Anabolic Process • Anabolism: metabolic pathway that stores energy by the synthesis of complex molecules form simple ones • Sunlight Energy + 6 CO2 + 6 H2O ---> C6H12O6 + 6 O2 + + Cellular Respiration as a Catabolic Process • Catabolism: metabolic pathway that releases energy by breaking down complex molecules into simpler compounds C6H12O6* + 6 O2 ------> 6 CO2 + 6 H2O + Energy (ATP + Heat) * carbohydrates, fats and proteins could all be processed in replacement of glucose + + Respiration as Combustion • You can think of cellular respiration as the combustion of gases in the car’s engine. • Main Engine: Mitochondria • Main Fuel: Glucose • Main Exhausts: CO2 + H2O ATP • The goal of cellular respiration is to generate ATP • WHAT IS ATP? • • • • Universal energy molecule Makes energy readily available Continuously being remade Stands for Adenosine Triphosphate Adenosine P P + P High Energy Bond Adenosine ATP Uses • What is ATP used for? – Movement: muscle contraction – Heat: hypothermia – Synthesis: hair, blood cells, fingernails – Active Transport: nervous system, kidneys – Bioluminescence: the generation of light using biochemical processes – Cellular Growth: energy for regular cellular processes Site of Cellular Respiration: Mitochondria • The primary site of cellular respiration is the mitochondria (cell organelle). • Glycolysis: occurs in the cytosol • Krebs Cycle: occurs in the mitochondrial matrix • Oxidative Phosphorylation: occurs in the inner membrane of the mitochondria Mitochondria • Components of a mitochondrion. Evolution of Mitochondria • Prokaryotes: cells that are small in size and have relatively simple construction. • Eukaryotes: cells that are large in size and have a complex construction. • There are many features that separate the two classifications. One characteristic is that prokaryotes do not have mitochondria. Picture of a eukaryote Endosymbiotic Theory • For a long period of Earth’s history only prokaryotes existed. • As prokaryotes developed they became increasingly specialized, living in adapted communities. • It is now theorized that specialized aerobic heterotrophic prokaryotes (mitochondria) began living inside larger host cells. Endosymbiotic Theory • These early mitochondria probably gained entry as undigested prey or as internal parasites. • Eventually this symbiosis became mutually beneficial. • It is also believed that chloroplasts developed in this manner. However, because not all eukaryotes have chloroplasts it is believed that chloroplasts began symbiotic processes after the development of mitochondria. Evidence • Existing symbiotic relationships. • Similarities between bacteria and the eukaryotic organelles of chloroplasts and mitochondria. For example: – – – – – – size construction of their membranes organelles split similar to binary fission in bacteria have circular DNA have their own ribosomes these ribosomes resemble prokaryotic ribosomes. Leber’s Disease There are 2 types of respiration 1 Aerobic Respiration – takes place in the presence of oxygen 2 Anaerobic Respiration– takes place in the absence of oxygen. Glycolysis • Glcolysis occurs in the cytosol of the cell. 1st Step of Cellular Respiration: Glycolysis • Glycolysis – Occurs in CYTOPLASM – Breaks GLUCOSE into two molecules called PYRUVIC ACID – Takes place in the ABSENCE OF OXYGEN 1st Step of Cellular Respiration: Glycolysis 1st Step of Cellular Respiration: Glycolysis • Glycolysis literally means “splitting of sugar”. • Notice that we have not yet used any oxygen. • Two ATP molecules are used up in the process, but four are produced. – A net gain of 2 ATP/glucose • Two molecules of NAD+ are reduced to NADH therefore – 2 NADH are produced/glucose Krebs Cycle • The Krebs cycle occurs in the mitochondrial matrix 2nd Step of Cellular Respiration: Krebs Cycle • Krebs Cycle – Occurs in mitochondrial matrix (inside the mitochondrion) – Completely decomposes pyruvic acid into CO2 – Needs oxygen to occur Krebs Cycle Krebs Cycle • 8 steps to produce – – – – 8 NADH/glucose 2 FADH/glucose 2 ATP/glucose 6 CO2/glucose • 2 Pyruvic acid molecules are changed into 6 CO2 molecules Electron Transport Chain • The electron transport chain is located on the inner membrane of the mitochondria 3rd Step of Cellular Respiration: Electron Transport Chain • Very small amounts of ATP have been generated so far, most will come from the electron transport chain. • Electron Transport Chain – Occurs on the inner membrane of the mitochondrion – Involves a group of molecules built into the inner membrane of the mitochondrion – Electrons (from NADH, FADH) pulled off of food by glycolysis and Krebs are passed between these molecules • This will ultimately result in the production of ATP – Oxygen is required for this step – Water is an end product – Lots of ATP is made (32) Electron Transport Chain • NADH transfers electrons to the first molecule in the electron transport chain. • These high energy electrons pass to successive molecules lowering their energy levels. • Reduction: occurs when the electrons are transported to successive electron carriers. • Oxidation: the state of electron carriers after an electron has been passed to the next carrier. Electron Transport Chain • Chemiosmosis: How the mitochondrial membrane couples electron transport to oxidative phosphorylation. Electron Transport Chain • At the same time H+ ions from the mitochondrial matrix are pumped across the inner membrane into the intermembrane space. • The result is a H+ gradient. Electron Transport Chain • Throughout the inner membrane of the mitochondrion there are proteins called ATP synthase. • ATP synthase creates ATP by using the hydrogen ion gradient. When ions cross the membrane the process is called chemiosmosis. • This gradient powers the process of oxidative phosphorylation. • Oxidative Phosphorylation: The production of ATP using energy derived from the redox reactions of an electron transport chain. Electron Transport Chain • The result is 3 ATP produced for every NADH molecule and 2 ATP produced for every molecule of FADH. Or, in total around 32 ATP are produced per glucose molecule in oxidative phosphorylation. Electron Transport Chain • The final step in the electron transport chain is the transfer of H+ ions to oxygen in the formation of water. • In other words, oxygen is the final electron acceptor during aerobic respiration. Big Picture: Why Electron Transport Chain? • The object of the ETC is to turn high energy electrons into low energy electrons. • If all this energy is released in one step, it would be lost as heat. • When it is released in many steps, it is converted into ATP. Anaerobic Respiration • The production of energy without oxygen. • Takes place in the cytoplasm. • Very inefficient -- only 6% of glucose energy is made available. • Only 2 ATP are produced per glucose molecule (vs. 36 in aerobic respiration) • Oxygen is required for pyruvic acid to enter Krebs where it can be broken down. Anaerobic Respiration • Pyruvic acid is toxic to the cell. When it does not enter the Krebs cycle it must be converted into a safer form. – In animals -- LACTIC ACID* • causes muscle cramps • process is called Lactic Acid Fermentation – In bacteria and yeast -- ETHYL ALCOHOL & CO2* • process is called Alcohol Fermentation * also denotes the final electron acceptors in anaerobic respiration Anaerobic Respiration • Notice how the NADH passes the electrons to the organic molecules. Versatility of Catabolism • Glycolysis can accept a wide range of carbohydrates for catabolism. This process begins when carbohydrates are hydrolyzed to glucose. • Proteins can be used but must be broken down into their constituent amino acids. They then loose their amino groups and become intermediates of glycolysis and the Krebs cycle. • Energy can also be harvested from fats. The glycerol of fats can be converted to an intermediate of glycolysis. Larger fatty acids can also be broken down into acetyl CoA. A gram of fat can generate twice as much ATP as a gram of carbohydrates. Versatility of Catabolism Pollutants effect on Respiration • Cyanide (CN-) blocks electron transport and therefore halts oxygen consumption. • Rotenone (Amazonian Aboriginals), comes from plants and is used as a toxin. Pollutants Effects on Cellular Respiration • Carbon monoxide is produced as a by-product in the combustion of organic molecules. • Carbon monoxide prevents cellular respiration when it binds to hemoglobin preventing the binding of oxygen. Effects of Pollutants on Cellular Respiration • Fluoride: Sodium fluoroacetate and fluoroacetamide are readily absorbed by the gut, but only to a limited extent across skin. • Three molecules of fluoroacetate or fluoroacetamide are combined in the liver to form a molecule of fluorocitrate. • This blocks the Krebs cycle inhibiting aerobic cellular respiration. • Fluorocitrate was found in the effluent of a factory and killed cows downstream.