biochem ch 34 [9-2
advertisement

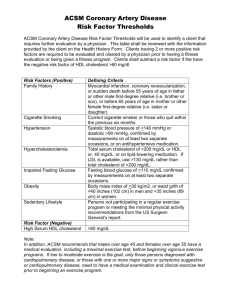
Cholesterol Overview Cholesterol – transported in the blood in lipoproteins because it is absolutely insoluble in water o Serves as stabilizing component of cell membranes and as precursor of bile salts as well as steroids Precursors of cholesterol converted to ubiquinone, dolichol, and (in the skin) cholecalciferol (active form of vitamin D) As major component of blood lipoproteins, cholesterol can appear in its free, unesterified form in outer shell of macromolecules and as cholesterol esters in lipoprotein core Precursor for cholesterol synthesis is acetyl coenzyme A (acetyl-CoA), which can be produced from glucose, fatty acids, or amino acids o 2 acetyl-CoA molecules form acetoacetyl-CoA, which condenses with another acetyl-CoA to form hydroxymethylglutaryl-CoA (HMG-CoA) o Reduction of HMG-CoA produces mevalonate (catalyzed by HMG-CoA reductase – rate-limiting step) o Mevalonate produces isoprene units that condense, eventually forming squalene o Cyclization of squalene produces the steroid ring system, and several subsequent reactions generate cholesterol Adrenal cortex, gonads, liver, and intestine all produce cholesterol in significant quantities, but the process can be found in any cell in the body Cholesterol is packaged in chylomicrons in intestine and VLDL in the liver and is transported in these forms along with triacylglycerol o As triacylglycerols of blood lipoproteins digested by lipoprotein lipase, chylomicrons are converted to chylomicron remnants, and VLDL is converted to intermediate-density lipoprotein (IDL) and subsequently LDL o Above products return to liver, where they bind to receptors and are taken up into vesicles which will then be digested by lysosomal enzymes o Cholesterol and other products of lysosomal digestion released into cellular pools o Liver uses recycled cholesterol and cholesterol synthesized from acetyl-CoA to produce VLDL and bile salts o Intracellular cholesterol obtained from blood lipoproteins decreases synthesis of cholesterol within cells, stimulates storage of cholesterol as cholesterol esters, and decreases synthesis of LDL receptors HDL – contains triacylglycerols and cholesterol – exchanges proteins and lipids with other lipoproteins in blood o Transfers apolipoprotein E (apo E) and apo CII to chylomicrons and VLDL o After digestion of VLDL triacylglycerols, apo E and apo CII transferred back to HDL o HDL obtains cholesterol from other lipoproteins and from cell membranes and converts it to cholesterol esters by lecithin-cholesterol acyltransferase (LCAT) reaction After the above, HDL either directly transports cholesterol and cholesterol esters to the liver or transfers cholesterol esters to other lipoproteins via the cholesterol ester transfer protein (CETP) o Lipoprotein particles carry the cholesterol and cholesterol esters to the liver, where endocytosis and lysosomal digestion occur o Reverse cholesterol transport (above return of cholesterol to liver) is major function of HDL Elevated levels of cholesterol in blood associated with formation of atherosclerotic plaques o High levels of LDL are especially atherogenic o High levels of HDL are protective because HDL particles are involved in process of removing cholesterol from tissues and returning it to liver Bile salts emulsify dietary triacylglycerols – digestive products absorbed by intestinal epithelial cells form bile salt micells (tiny microdroplets that contain bile salts at their water interface) o After contents of micells are absorbed, most bile salts travel to the ileum, where they are resorbed and recycled by liver Fecal excretion of bile salts is major means by which body disposes of steroid nucleus of cholesterol o Because ring structure of cholesterol cannot be degraded in body, it is excreted mainly in bile as free cholesterol and bile salts Intestinal Absorption of Cholesterol Key regulatory point in sterol metabolism because it ultimately determines what percentage of biliary cholesterol produced by liver each day and what percentage of dietary cholesterol entering gut per day is eventually absorbed into the blood o In normal people, about 55% of intestinal pool enters blood through enterocytes each day Unwanted or excessive cholesterol is removed and sterols from enterocyte are planted o ATP-binding cassette (ABC) protein family, specifically ABCG5 and ABCG8 couple ATP hydrolysis to transport of unwanted or excessive cholesterol and plant sterols (phytosterols) from enterocyte back into gut lumen ABCA1 is required for reverse cholesterol transport and biogenesis of HDL ABC protein expression increases amount of sterols present in gut lumen, with potential to increase elimination of sterols into feces Phytosterolemia – rare autosomal recessive disease also called sitosterloemia – defect in function of either ABCG5 or ABCG8 in enterocytes, which leads to accumulation of cholesterol and phytosterols in these cells – eventually reaches bloodstream, markedly elevating level of cholesterol and phytosterol in blood, causing increased cardiovascular morbidity Ezetimibe – structurally different from sterols – primary action in lowering serum cholesterol levels is to block cholesterol absorption through specific cholesterol absorption mechanism in brush border of enterocytes o Target is Niemann-Pick C1-like 1 (NPC1L1) protein, which transports cholesterol into cells via absorptive endocytotic mechanism (clathrin-dependent) Cholesterol Synthesis Cholesterol – alicyclic compound whose basic structure includes perhydrocyclopentanophenanthrene nucleus containing four fused rings (3 cyclohexanes and 1 cyclopentane) About 1/3 of cholesterol exists in free (unesterified) form, and the other 2/3 exist as cholesterol esters in which a long-chain fatty acid (usually linoleic acid) is attached by ester linkage to OH at C3 (bottom left corner of above picture) o Percentage of free and esterified cholesterol can be measured using highperformance liquid chromatography (HPLC) Cholesterol Synthesis Stage 1: Synthesis of Mevalonate from Acetyl-CoA Committed, rate-limiting step in cholesterol formation HMG-CoA synthase present in cytosol and distinct from mitochondrial HMG-CoA synthase that catalyzes HMG-CoA synthesis involved in production of ketone bodies Committed step and major point of regulation is reduction of HMG-CoA to mevalonate (last step in picture to right) o HMG-CoA reductase embedded in membrane of ER and contains 8 membranespanning domains – amino-terminal domain faces cytoplasm and contains enzymatic activity Rate of synthesis of HMG-CoA reductase mRNA controlled by a member of sterolregulatory element-binding protein family (SREBPs) o Transcription factors belong to basic helix-loop-helix leucine zipper (bHLH-Zip) family of transcription factors that directly activate the expression of more than 30 genes dedicated to synthesis and uptake of cholesterol, fatty acids, triacylglycerols, and phospholipids as well as production of NADPH cofactors required to synthesize these molecules o SREBPs specifically enhance transcription of HMG-CoA reductase gene by binding to sterol-regulatory element (SRE) upstream of the gene – when bound, rate of transcription increases o SREBPs, after synthesis, are integral proteins of ER SREBP is bound to SREBP cleavage-activating protein (SCAP) in ER membrane when cholesterol levels are high When cholesterol levels drop, sterol leaves SCAP-binding site and SREBP-SCAP complex is transported to Golgi apparatus In Golgi, 2 proteolytic cleaves occur (via site 1 and site 2 proteases [S1P and S2P]), which release N-terminal transcription factor domain from Golgi membrane Once released, active amino terminal component travels to nucleus to bind to SREs o Soluble SREBPs are rapidly turned over and need to be continuously produced to stimulate reductase mRNA transcription effectively o When cytoplasmic sterol levels rise, sterols bind to SCAP and prevent translocation of complex to Golgi, leading to decrease in transcription of reductase gene and thus less reductase protein being produced Rising levels of cholesterol and bile salts in cells that synthesize above molecules may cause change in oligomerization state of membrane domain of HMG-CoA reductase, rendering the enzyme more susceptible to proteolysis, decreasing its activity o Membrane domains of HMG-CoA reductase contain sterol-sensing regions similar to those in SCAP Activity of reductase regulated by phosphorylation and dephosphorylation o Elevated glucagon levels increase phosphorylation of HMG-CoA reductase, inactivating it o Hyperinsulinemia increases activity of reductase by activating phosphatases, which dephosphorylate HMG-CoA reductase o Increased levels of intracellular sterols increase phosphorylation of HMG-CoA reductase (feedback suppression) o Thyroid hormone increases HMG-CoA activity o Glucocorticoids decrease HMG-CoA activity o AMP-activated protein kinase is the enzyme that actually phosphorylates HMG-CoA reductase AMP-activated protein kinase is regulated by phosphorylation by LKB1 o Cholesterol synthesis decreases when ATP levels low and increases when ATP levels high Cholesterol Synthesis Stage 2: Conversion of Mevalonate to Two Activate Isoprenes Purpose of phosphate transfers is to activate both C5 and hydroxyl group on C3 for further reactions o Phosphate group attached to C3 hydroxyl group of mevalonate in 3phospho-5-phyrophosphomevalonate intermediate is removed along with carboxyl group on C1, producing the double bond in the second to last step (see pathway on previous page) The bottom two products are the isoprenes – also used in synthesis of coenzyme Q and dolichol Cholesterol Synthesis Stage 3: Condensation of 6 Activated 5-Carbon Isoprenes to Form the 30-Carbon Squalene Head-to-tail condensation of isopentenyl pyrophosphate and dimethylallyl pyrophosphate o “head” is end of molecule to which pyrophosphate is linked Geranyl pyrophosphate undergoes another head-to-tail condensation with isopentenyl pyrophosphate 2 molecules of farnesyl pyrophosphate undergo a head-to-head fusion, and both pyrophosphate groups are removed to form squalene Geranyl pyrophosphate and farnesyl pyrophosphate are key components in cholesterol biosynthesis and both can form covalent bonds with proteins, particularly G proteins and certain proto-oncogene products involved in signal transduction o Hydrophobic groups anchor proteins in cell membrane Cholesterol Synthesis Stage 4: Conversion of Squalene to the Four-Ring Steroid Nucleus When squalene monooxygenase adds a single O from O2 to the squalene molecule, NADPH reduces the other O to H2O The “many reactions” are a series of complex reactions, containing many steps, elucidated in the late 1950’s Several Fates of Cholesterol Placenta in pregnant women can also produce cholesterol Fraction of hepatic cholesterol used for synthesis of hepatic membranes, but bulk is secreted from hepatocyte as o Cholesterol esters o Biliary cholesterol (cholesterol found in bile) o Bile acids Cholesterol ester production in liver catalyzed by acyl-CoA-cholesterol acyl transferase (ACAT), which catalyzes the transfer of fatty acid from coenzyme A to hydroxyl group on C3 of cholesterol Cholesterol esters more hydrophobic than free cholesterol Liver packages some esterified cholesterol into hollow core of lipoproteins, primarily VLDL, which is secreted from hepatocyte into blood and transports cholesterol esters (triacylglycerols, phospholipids, apoproteins, etc.) to tissues that require greater amounts of cholesterol than they can synthesize de novo Tissues use cholesterol for synthesis of membranes, formation of steroid hormones, and biosynthesis of vitamin D o Residual cholesterol esters not used stored in liver for later use Hepatic cholesterol pool serves as source of cholesterol for synthesis of hydrophilic bile acids and their salts – very effective detergents because they contain both polar and nonpolar regions o Aid in digestion of intraluminal lipids by forming micelles with them, which increases surface area of lipids exposed to digestive action of intraluminal lipases Free cholesterol also enters gut lumen via biliary tract, forming an intestinal pool, 55% of which is resorbed by enterocytes and enters bloodstream Greater intake of dietary cholesterol suppresses rate of hepatic cholesterol synthesis (feedback repression) Synthesis of Bile Salts Bile salts synthesized by reactions that hydroxylate the steroid nucleus and cleave the side chain First step is rate-limiting reaction (shown to right) o Activity of 7-α-hydroxylase decreased by an increase in bile salt concentration pKa of bile acids is ~6 o Contents of intestinal lumen usually around pH 6, so about 50% of molecules present are protonated, and 50% are ionized (forming bile salts) second step of cholesterol conversion Carboxyl group at end of side chain of bile salts activated by a reaction that requires ATP and CoA derivatives that can react with either glycine or taurine (which is derived from cysteine), forming amides (conjugated bile salts) o Glycine conjugated derivatives have a pKa of about 4 o Taurine conjugates have pKa of about 2 Fate of Bile Salts Bile salts serve as detergents that aid in digestion of dietary lipids Intestinal bacteria deconjugate and dehydroxylate bile salts, removing the glycine and taurine residues and the hydroxyl group at position 7 Bile salts that lack a hydroxyl group at position 7 are secondary bile salts Deconjugated and dehydroxylated bile salts are less soluble and thus less readily resorbed from intestinal lumen – they often get excreted Greater than 95% of bile salts are resorbed in ileum and return to liver via enterohepatic circulation (portal vein) Secondary bile salts may be reconjugated in liver, but they are not rehydroxylated Because steroid nucleus cannot be degraded by body, excretion of bile salts serves as major route for removal of steroid nucleus, and thus cholesterol, from body Transport of Cholesterol by Blood Lipoproteins Cholesterol and cholesterol esters transported through bloodstream packaged as lipoproteins, where are composed of a core of hydrophobic lipids such as cholesterol esters and triacylglycerols surrounded by a shell of polar lipids (phospholipids), which allows hydration shell to form around the lipoprotein o Positive charge of nitrogen atom of phospholipid forms ionic bond with negatively charged hydroxyl ion of the environment Shell of lipoprotein contains variety of apoproteins that increase water solubility of lipoprotein Free cholesterol molecules dispersed throughout lipoprotein shell to stabilize it in a way that allows it to maintain its spherical shape Lipoproteins are transported to tissues, where their components are either used in synthetic or oxidative processes or stored for later use Apoproteins – “apo” describes protein within the shell of the particle in its lipid-free form Apoproteins add to hydrophilicity as well as structural stability of the particle o Activate certain enzymes required for normal lipoprotein metabolism o Act as ligands on surface of lipoprotein that target specific receptors on peripheral tissues that require lipoprotein delivery for their innate cellular functions Chylomicrons – largest of lipoproteins and least dense because of rich triacylglycerol content o Synthesized from dietary lipids (exogenous lipoprotein pathway) within epithelial cells of small intestine and secreted into lymphatic vessels draining gut o apoCII activates lipoprotein lipase (LPL), an enzyme that projects into lumen of capillaries in adipose tissue, cardiac muscle, skeletal muscle, and acinar cells of mammary tissue, allowing LPL to hydrolyze chylomicrons, leading to release of free fatty acids derived from core triacylglycerides of lipoprotein into target cells o Muscle cells oxidize fatty acids as fuel, and the other cells store them as triacylglycerols (fat) or mammary cells use them for milk formation o Following the chylomicron remnants, receptors in PM of liver cells bind to apoE on surface of remnants, allowing them to be taken up by liver VLDL – packaged forms of excess carbohydrates that have been converted to triacylglycerols – also have free and esterified cholesterol, phospholipids, and apoB100 o Above particles secreted from liver (endogenous pathway of lipoprotein metabolism) into blood stream, where they accept apoCII and apoE from circulating HDL particles, forming the mature VLDL particle o VLDL particles transported from hepatic veins to capillaries in skeletal and cardiac muscle and adipose tissue, as well as lactating mammary tissues, where LPL is activated by apoCII in VLDL particles o Activated LPL facilitates hydrolysis of triacylglycerol in VLDL, causing release of fatty acids and glycerol from portion of core triacylglycerols o Fatty acids released here oxidized as fuel by muscle cells, used in resynthesis of triacylglycerols in fat cells, and used for milk production in lactating breast o Residual particles are VLDL remnants – about 50% of these are taken up by liver cells through binding of VLDL apoE to hepatocyte PM receptors, followed by endocytotic internalization of VLDL remnant The half of VLDL remnants not taken up by liver have additional core triacylglycerols removed to form IDL o With removal of additional triacylglycerols from IDL through action of hepatic triglyceride lipase in hepatic sinusoids, LDL is made from IDL o About 60% of LDL transported back to liver, where apoB100 binds to receptors in liver PM, allowing particles to be endocytosed o Remaining 40% of LDL carried to extrahepatic tissues such as adrenocortical and gonadal cells that also have apoB100 receptors, allowing them to internalize the LDL particles and use their cholesterol for synthesis of steroids Some of internalized LDL used for membrane synthesis and vitamin D synthesis as well o If excess of LDL particles present in blood, receptor-mediated uptake of LDL by tissue becomes saturated and the excess LDL particles are more readily available for nonspecific uptake of LDL by macrophages present near endothelial cells of arteries Exposure of vascular endothelial cells to high levels of LDL induces an inflammatory response by these cells, initiating cascade of atherosclerosis HDL – plays several roles in whole-body lipid metabolism o HDL can be created by Synthesis of nascent HDL by liver and intestine as relatively small molecule, whose shell contains phospholipids, free cholesterol, and variety of apoproteins – very low levels of triacylglycerols or cholesterol esters found in hollow core of early (nascent) HDL Budding apoproteins from chylomicrons and VLDL particles as they are digested by LPL – apoproteins and shells can accumulate more lipid o o o o Free apoAI, which may be shed from other circulating lipoproteins, acquires cholesterol and phospholipids from other lipoproteins and cell membranes, forming nascent-like HDL particle within circulation In process of maturation, nascent HDL particles accumulate phospholipids and cholesterol from cells lining blood vessels As central hollow core of nascent HDL fills with cholesterol esters, HDL takes on a more globular shape to eventually form the mature HDL particle Transfer of lipids to nascent HDL does not require enzyme activity HDL particles can remove cholesterol from cholesterol-laden cells and return it to the liver (reverse cholesterol transport) By reducing cellular cholesterol levels in subintimal space of blood vessels, the likelihood that foam cells will form in vessel wall is reduced Reverse cholesterol transport requires directional movement of cholesterol from cell to lipoprotein particle Cells contain protein ABCA1 (ATP-binding cassette protein 1) that uses ATP hydrolysis to move cholesterol from inner leaflet of membrane to outer leaflet Once cholesterol has reached outer leaflet, HDL particle can accept it, but if cholesterol not modified within HDL particle, cholesterol can leave particle by same route it entered To trap cholesterol within HDL core, the HDL particle acquires enzyme lecithin-cholesterol acyltransferase (LCAT) from circulation (LCAT is synthesized and secreted by liver) Cholesterol ester from above migrates to core of HDL particle and is no longer free to return to cell Elevated levels of lipoprotein-associated cholesterol in blood, particularly that associated with LDL and more triacylglycerol-rich lipoproteins, are associated with formation of cholesterol-rich atherosclerotic plaque in vessel wall High levels of HDL in blood are vasculoprotective because high levels increase rate of reverse cholesterol transport away from blood vessels and toward liver Mature HDL particles can bind to specific receptors on hepatocytes, but primary means of clearance of HDL from blood is through uptake by scavenger receptor SR-B1 present on many cell types Once HDL particle is bound to the receptor, its cholesterol and cholesterol esters are transferred into the cells When depleted of cholesterol and its esters, HDL particle dissociates from SR-B1 receptor and reenters circulation SR-B1 receptors can be upregulated in certain cell types that require cholesterol for biosynthetic purposes, such as steroid-producing cells SR-B1 receptors not downregulated when cholesterol levels are high HDL exchanges apoproteins and lipids with other lipoproteins in blood (apoE and apoCII go to chylomicrons and VLDL) apoCII stimulates degradation of triacylglycerols of chylomicrons and VLDL by activating LPL After digestion of chylomicrons and VLDL triacylglycerols, apoE and apoCII are transferred back to HDL When HDL obtains free cholesterol from PM’s, free cholesterol is esterified at C3 of the A-ring via the LCAT reaction HDL either transports free cholesterol and its esters directly to the liver or by CETP to circulating triacylglycerol-rich lipoproteins such as VLDL and VLDL remnants – in exchange, triacylglycerols from latter lipoproteins transferred to HDL The greater the concentration of triacylglycerol-rich lipoproteins in blood, the greater the rate of the above exchanges – thus, whenever triacylglycerol-rich lipoproteins present in blood in high concentrations, amount of cholesterol reaching liver via cholesterol-enriched VLDL and VLDL remnants increases, and proportional reduction in total amount of cholesterol and cholesterol esters that are transferred directly to the liver via HDL occurs o Nomenclature of HDL Mature HDL particles HDL3 After reverse cholesterol transport and accumulation of cholesterol esters HDL2 (atherogenic protective form) CETP reaction leads to loss of cholesterol and gain of triacylglycerol, such that particles become larger and become HDL3 again Hepatic lipase can remove triacylglycerol from HDL3 particles to form HDL2 Lipoproteins Enter Cells by Receptor-Mediated Endocytosis When an apoproteins attaches to an LDL receptor on a cell membrane, it is endocytosed via clathrin-dependent endocytosis Endocytosed vesicles fuse with lysosomes, where the cholesterol esters of LDL are hydrolyzed to form free cholesterol, which is re-esterified through action of ACAT – rapid re-esterification necessary to avoid damaging effect of high levels of free cholesterol on cellular membranes o Newly esterified cholesterol contains primarily oleate or palmitoleate (monounsaturated fatty acids), unlike those of cholesterol esters in LDL, which are rich in linoleate (polyunsaturated fatty acid) Synthesis of LDL receptor is subject to feedback inhibition by increasing levels of cholesterol within cell o SREBPs or cofactors required for full expression of genes that code for LDL receptor capable of sensing concentration of sterols within cell o When sterol levels high, the process leading to binding of SREBP to SRE of LDL receptor is diminished, reducing amount of cholesterol that can enter the cell o When intracellular levels of cholesterol decrease, the above processes are reversed, and cells act to increase their cholesterol levels (synthesis of cholesterol from acetyl-CoA and synthesis of LDL receptors stimulated) Lipoprotein Receptors Best characterized lipoprotein receptor (LDL receptor) recognizes apoB100 and apoE, so it binds VLDL, IDL, LDL, and chylomicron remnants o Binding reaction characterized by saturability and occurs with high affinity and narrow range of specificity LDL receptor-related proteins (LRPs) and macrophage scavenger receptor (SR-A1 and SR-A2 near endothelial surface of vascular endothelial cells) have broad specificity and bind many other ligands in addition to blood lipoproteins LDL receptor – has mosaic structure encoded by gene assembled by exon shuffling o Located in short arm of chromosome 19 o Protein encoded by gene has 6 regions Amino terminus has LDL-binding region – acidic side chains bind Ca2+ – when these side chains are protonated, calcium is released from its binding sites, leading to conformational changes that allow LDL to dissociate from its receptor docking site Disulfide bonds, formed from cysteine residues, have stabilizing influence on structural integrity of this portion Next region contains domains homologous with EGF as well as with complex consisting of 6 repeats that resemble the blades of the transducing β-subunit, forming a propeller-like moiety Next region contains chain of N-linked oligosaccharides Next region contains domain rich in serine and threonine and contains O-linked sugars – may have role in physically extending receptor away from membrane so LDL-binding region is accessible to LDL molecule Next region contains 22 hydrophobic residues that constitute membrane-spanning unit of the receptor Last region extends into the cytosol, where it regulates the interaction between the C-terminal domain of the LDL receptor and the clathrin-containing coated pit where the process of receptor-mediated endocytosis is initiated o LDL receptors and their binding ability can be diminished by mutations in one or both alleles for LDL receptor (familial hypercholesterolemia) Heterozygotes produce half the normal LDL receptors, and homozygotes produce almost none 4 classes of mutations identified Null alleles that either direct synthesis of no protein at all or a protein that cannot be precipitated by antibodies to the LDL receptor Alleles encode proteins, but they cannot be transported to cell surface Mutant alleles encode proteins that reach cell surface but cannot bind LDL normally Gene encodes proteins that reach surface and bind LDL but fail to cluster and internalize LDL particles LRP receptors – structurally related to LDL receptor but recognizes broader spectrum of ligands o Can bind lipoproteins, α2-macroglobulin (protein that inhibits blood proteases) and tissue plasminogen activator (TPA) and its inhibitors o LRP receptor recognizes apoE of lipoproteins and binds remnants produced by digestion of triacylglycerols of chylomicrons and VLDL by LPL, thus clearing these remnants from the blood stream o LRP receptor is abundant in cell membranes in liver, brain, and placenta o Synthesis of LRP receptor not significantly affected by increase in intracellular concentration of cholesterol o Insulin causes number of LRP receptors on cell surface to increase Macrophage scavenger receptor – nonspecific receptors that bind various types of molecules, including oxidatively modified LDL particles o SR-B1 – used primarily for HDL binding o SR-A1 and SR-A2 – scavenger receptors o Modification of LDL frequently involves oxidative damage, particularly of polyunsaturated fatty acyl groups o Not subject to downregulation o Continued presence of scavenger receptors in cell membrane allows cells to take up oxidatively modified LDL long after intracellular cholesterol levels are elevated o Macrophages engorged with lipid are foam cells, which accumulates in fatty streaks (precursors to atherosclerotic plaques) o Processes that cause oxidation of LDL involve superoxide radicals, nitric oxide, H2O2, and other oxidants o Antioxidants such as vitamin E, ascorbic acid, and carotenoids may protect LDL from oxidation Anatomic and Biochemical Aspects of Atherosclerosis Initial step in development of atherosclerotic lesion is formation of a fatty streak o Fatty streak – accumulation of lipid-laden macrophages (foam cells) in subintimal space o Fatty streaks visible as yellow-white linear streak that bulges slightly into lumen of vessel o Streaks initiated when one or more known vascular risk factors for atherosclerosis, all of which have potential to injure the vascular endothelial cells, reach critical threshold at site of future lesions Risk factors include Arterial hypertension Elevated circulating levels of LDL, chylomicron remnants, and VLDL remnants Low levels of circulating HDL Cigarette smoking Chronic elevations in blood glucose levels High circulating levels of vasoconstricting angiotensin II Resulting insult to endothelial cells may trigger injured cells to secrete adhesion molecules that bind to circulating monocytes and markedly slow their rate of movement past the endothelium – when monocytic cells slowed enough, they accumulate and have access to physical spaces that exist between endothelial cells, resembling the classical inflammatory response to injury Monocytic cells transformed into macrophages that migrate through spaces between endothelial cells, entering subintimal space under influence of chemoattractant cytokines secreted by vascular cells in response to exposure to oxidatively modified fatty acids within lipoproteins Macrophages can replicate and exhibit augmented expression of receptors that recognize oxidatively modified lipoproteins – receptors are high-capacity, low-specificity receptors that bind to and internalize oxidatively modified fatty acids within LDLs to become subintimal foam cells As foam cells accumulate, they deform the overlying endothelium, causing microscopic separations between endothelial cells, exposing foam cells and the underlying extracellular matrix to blood – exposed areas serve as sites for platelet adhesion and aggregation Activated platelets secrete cytokines that perpetuate this process and increase potential for thrombus formation locally As evolving plaque matures, fibrous cap forms over its expanding “roof”, which bulges into the vascular lumen, partially occluding it Vascular smooth muscle cells migrate from tunica media to subintimal space and secrete additional plaque matrix material and metalloproteinases that thin the fibrous cap near its “elbow” at the periphery of the plaque o Thinning progresses until fibrous cap ruptures, allowing plaque contents to physically contact procoagulant elements present in circulation, leading to acute thrombus formation Most plaques that rupture also contain focal areas of calcification, resulting from induction of same cluster of genes as those that promote formation of bone o Inducers for this process include oxidized sterols and transforming growth factor β (TGF-β) derived from certain vascular cells High intraluminal sheer forces develop in the thinning or eroded areas of plaque’s fibrous cap, inducing macrophages to secrete additional metalloproteinases that further degrade the arterial-fibrous cap matrix, contributing further to plaque rupture and thrombus formation Steroid Hormones Steroid hormones, because of their hydrophobicity, cross cell membrane and bind to specific receptors in either the cytoplasm or nucleus o Bound receptors bind to DNA to regulate gene transcription Because of hydrophobicity, steroids must be complexed with serum protein o Albumin can act as a nonspecific carrier, but there are specific carriers as well Cholesterol used for steroid hormone synthesis is synthesized in tissues from acetyl-CoA, extracted from intracellular cholesterol ester pools, or taken up by cell as cholesterol-containing lipoproteins Specific complement of enzymes present in cells of an organ determines which hormones the organ can synthesize Androgens such as testosterone are synthesized in Leydig cells (and to a lesser extent the ovary) and secreted in response to LH produced in the anterior pituitary gland o In men, testosterone is commonly converted to dihydrotestosterone (DHT), a higher affinity form of the hormone, within specific target tissues o Active form of hormone stimulates production of sperm proteins in Sertoli cells and development of secondary sex characteristics Estrogens such as 17-β-estradiol are synthesized in the ovarian follicle and corpus luteum, and secretion of them is stimulated by FSH produced in anterior pituitary o 17-β-estradiol has a negative feedback on synthesis and secretion of pituitary gonadotropins, such as FSH Progestogens such as progesterone synthesized in corpus luteum, and secretion stimulated by LH – progesterone prepares the uterine endometrium for implantation of fertilized ovum and acts as differentiation factor in mammary gland development Biosynthesis of glucocorticoids, mineralocorticoids, and sex steroids (of both adrenal cortex and gonads) requires several distinct cytochrome P450 enzymes o Monooxygenases involved in transfer of electrons from NADPH through electron-transfer protein intermediates to molecular oxygen, which then oxidizes a variety of ring carbons of cholesterol Cholesterol converted to progesterone in first 2 steps of synthesis of all steroid hormones o Cytochrome P450SCC side-chain cleavage enzyme (CYP11A) located in mitochondrial inner membrane Rate of biosynthesis of cortisol and other adrenal steroids depends on stimulation of adrenal cortical cells by ACTH o ACTH has permissive action on aldosterone production, allowing cells to respond optimally to their primary stimulus (angiotensin II) Adrenal androgens can be converted in other (not adrenal) tissues LH stimulates synthesis of testosterone and other androgens by Leydig cells in testicle o Predominant pathway is through pregnenolone to 17-αhydroxypregnenolone to DHEA, and then from DHEA to androstenedione to testosterone o Rate limiting step is conversion of cholesterol to pregenenolone o LH controls rate of side-chain cleaveage from cholesterol at C21 to form pregnenolone and thus regulates the rate of testosterone synthesis Ovarian production of estrogens, progestins, and androgens requires activity of cytochrome P450 family of oxidative enzymes used for synthesis of other steroid hormones o Major steroid producing compartments of ovary (granulosa cell, theca cell, stromal cell, and cells of corpus luteum) have all enzyme systems required for synthesis of multiple steroids, but granulosa cells secrete primarily estrogens, theca and stromal cells secrete primarily androgens, and cells of corpus luteum secrete primarily progesterone o Ovarian granulosa cell, in response to stimulation by FSH and catalytic activity of P450 aromatase (CYP19), converts testosterone to estradiol (predominant and most potent ovarian estrogen) o Androstenedione is converted to estrone in ovary, although major site of estrone production from androstenedione occurs in extraovarian tissues, principally skeletal muscle and adipose tissue Congenital Adrenal Hyperplasia Group of diseases caused by genetically determined deficiency in variety of enzymes required for cortisol synthesis Most common is deficiency is 21-α-hydroxylase (CYP21) – needed to convert progesterone to 11deoxycorticosterone and 17- α-hydroxyprogesterone to 11-deoxycortisol o Deficiency reduces both aldosterone and cortisol production without affecting androgen production 11-β-hydroxylase (CYP11B1) deficiency – results in accumulation of 11-deoxycorticosterone o Excess of mineralocorticoid leads to hypertension (through binding of 11-deoxycorticosterone to aldosterone receptor o 11-deoxycorticosterone also accumulates, but its biologic activity is minimal and no specific clinical signs and symptoms result from this o Androgen pathway unaffected, though increased ACTH may increase androgen levels in blood 17-α-hydroxlyase (CYP17) deficiency – leads to aldosterone excess and hypertension o Because adrenal androgen synthesis requires this enzyme, no virilization occurs in patients Vitamin D Synthesis Vitamin D can be obtained either from diet (vitamin D2 or D3) or synthesized from cholesterol precursor, requiring reactions in skin, liver, and kidney Calciferols, including several forms of vitamin D, are a family of steroids that affect calcium homeostasis o Cholecalciferol (vitamin D3) requires UV light for its production from 7-dehydrocholesterol present in cutaneous tissues – irradiation cleaves the carbon-carbon bond at C9-C10, opening the B-ring to form cholecalciferol, an inactive precursor of calcitriol, which is the most potent biologically active form of vitamin D Formation of calcitriol from cholecalciferol begins in liver and ends in kidney, where the pathway is regulated o C25 of vitamin D2 or D3 is hydroxylated in microsomes of liver to form calcidiol, which circulates to kidney bound to vitamin D-binding globulin (transcalciferin) o In proximal convoluted tubule, a mixed-function oxidase, which requires molecular O2 and NADPH, hydroxylates C1 on A-ring to form calcitriol (rate-limiting step and tightly regulated) o Release of PTH results in activation of the last step above 1,25-(OH)2D3 (calcitriol) is about 100x more potent than 25-(OH)D3, but 25-(OH)D3 is present in blood in concentration about 100x greater (plays a role in calcium and phosphorus homeostasis) Biologically active forms of vitamin D are sterol hormones that diffuse passively through PM o In intestine, bone, and kidney, sterol then moves into nucleus and binds to specific vitamin D3 receptors, activating genes that encode proteins mediating action of active vitamin D3 o In intestinal mucosal cell, transcription of genes that encode calcium-transporting proteins activated, carrying Ca2+ and phosphorus absorbed from gut lumen across cell, making it available for eventual passage into circulation Drugs Metformin – used to treat type 2 diabetes o Reduces blood glucose levels by inhibiting hepatic gluconeogenesis, which is active in these patients because of liver’s resistance to effects of insulin o Reduces lipid synthesis in liver, which aids in modulating blood lipid levels o Activates AMP-activated protein kinase (AMPK) through activation of an upstream protein kinase (LKB1) AMPK, when active, phosphorylates and reduces activity of acetyl-CoA carboxylase (required for fatty acid synthesis) and HMG-CoA reductase (reducing biosynthesis of cholesterol) Activation of AMPK activates glucose uptake by muscle, significant for reducing circulating blood glucose levels Activation of AMPK leads to cascade of transcriptional regulation that reduces liver’s ability to undergo both gluconeogenesis and lipogenesis Activated AMPK phosphorylates coactivator of CREB transcription factor (TORC2), which, when phosphorylated, is sequestered in cytoplasm and CREB becomes very inefficient at transcribing PGC1α, a gene required to upregulate genes that code for enzymes involved in gluconeogenesis, such as glucose 6-phosphatase and PEPCK o In presence of metformin, hepatic gluconeogenesis is reduced and muscle uptake of blood glucose is enhanced, leading to stabilization of blood glucose levels o Activation of AMPK also inhibits liver lipogenesis by decreasing transcription of key lipogenic enzymes, including fatty acid synthase and acetyl-CoA carboxylase Reduced transcriptional activity mediated by AMPK inhibition of transcription of sterolregulatory element-binding protein 1 (SREBP-1), which regulates transcription of HMG-CoA reductase and other lipogenic enzymes Fibrates – class of drugs used to decrease lipid levels (principally triglycerides) o Major target is PPARα – fibrate binding to it activates its transcription factor, which then leads to transcription of transport proteins (to enhance rate of fatty acid transport into cells), fatty acid translocase (increasing mitochondrial uptake of fatty acids), long-chain fatty acyl-CoA synthetase (activating fatty acids in cytoplasm), and carnitine palmitoyl transferase I (which enhances uptake of fatty acids into mitochondria) o PPARα also enhances lipoprotein lipase (LPL) expression, represses apoCIII expression (apoCIII inhibits apoCII activation of LPL), and stimulates apoAI and apoAII synthesis (major proteins in HDL) Thiazolidinediones (TZDs) – class of drug used for treatment of insulin resistance and type 2 diabetes mellitus o Activate PPARγ class of transcription factors, primarily expressed in adipose tissue o PPARγ in adipose is responsible for activating transcription of adiponectin, leading to increased circulating adiponectin levels o Increase in adiponectin reduces fat content of liver and enhances insulin sensitivity via AMPK-dependent pathway o TZDs also lead to reduction in plasma free fatty acid levels, which leads to enhanced insulin sensitivity