CDCP - 02.28.11 - Titration Excel Practice Example
advertisement
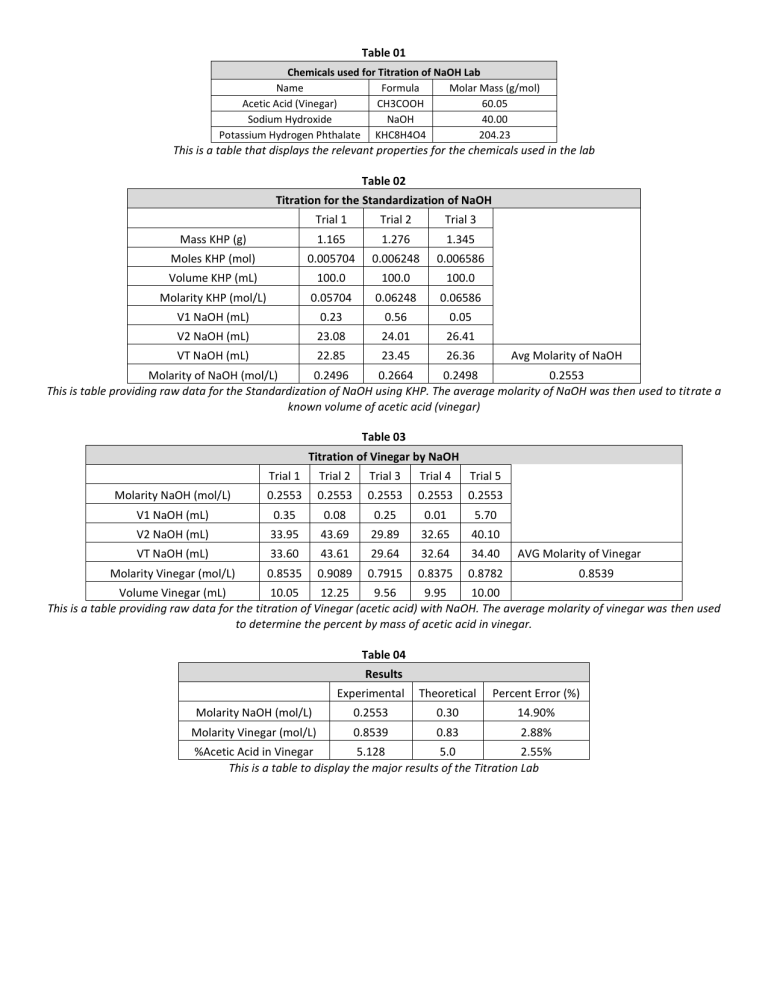
Table 01 Chemicals used for Titration of NaOH Lab Name Formula Molar Mass (g/mol) Acetic Acid (Vinegar) CH3COOH 60.05 Sodium Hydroxide NaOH 40.00 Potassium Hydrogen Phthalate KHC8H4O4 204.23 This is a table that displays the relevant properties for the chemicals used in the lab Table 02 Titration for the Standardization of NaOH Trial 1 Trial 2 Trial 3 Mass KHP (g) 1.165 1.276 1.345 Moles KHP (mol) 0.005704 0.006248 0.006586 Volume KHP (mL) 100.0 100.0 100.0 Molarity KHP (mol/L) 0.05704 0.06248 0.06586 V1 NaOH (mL) 0.23 0.56 0.05 V2 NaOH (mL) 23.08 24.01 26.41 VT NaOH (mL) 22.85 23.45 26.36 Avg Molarity of NaOH Molarity of NaOH (mol/L) 0.2496 0.2664 0.2498 0.2553 This is table providing raw data for the Standardization of NaOH using KHP. The average molarity of NaOH was then used to titrate a known volume of acetic acid (vinegar) Table 03 Titration of Vinegar by NaOH Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 Molarity NaOH (mol/L) 0.2553 0.2553 0.2553 0.2553 0.2553 V1 NaOH (mL) 0.35 0.08 0.25 0.01 5.70 V2 NaOH (mL) 33.95 43.69 29.89 32.65 40.10 VT NaOH (mL) 33.60 43.61 29.64 32.64 34.40 Molarity Vinegar (mol/L) 0.8535 0.9089 0.7915 0.8375 0.8782 AVG Molarity of Vinegar 0.8539 Volume Vinegar (mL) 10.05 12.25 9.56 9.95 10.00 This is a table providing raw data for the titration of Vinegar (acetic acid) with NaOH. The average molarity of vinegar was then used to determine the percent by mass of acetic acid in vinegar. Table 04 Results Experimental Theoretical Percent Error (%) Molarity NaOH (mol/L) 0.2553 0.30 14.90% Molarity Vinegar (mol/L) 0.8539 0.83 2.88% %Acetic Acid in Vinegar 5.128 5.0 2.55% This is a table to display the major results of the Titration Lab Table 05 Equation Example Explanation 𝑉𝑡 = 𝑉𝑓 − 𝑉𝑖 𝑉𝑖 = 0.04 𝑚𝐿 𝑉𝑓 = 34.56 𝑚𝐿 𝑉𝑡 = 34.56 − 0.04 = 34.52 𝑚𝐿 This calculation was used for finding the volume of NaOH dispensed by the buret in both the Standardizatio n and Neutralization The mass of KHP was measured out during the lab and dissolved into water, in order for the molarity to be calculated the #moles was needed The moles of solute dissolved in 1.0 L solution is the concentration (molarity) The titration equation is very similar to the dilution equation and is used to determine equivalent moles of each This is the simple average, calculated since 2nd grade The percent error is a display of the difference between the accepted and experimental values Volume Dispensed by buret Calcul ation 𝑚𝑜𝑙𝑒𝑠 = 𝑚𝑜𝑙𝑒𝑠 𝑔𝑟𝑎𝑚𝑠 20.00 𝑔 𝐻2𝑂 𝑥 1 𝑚𝑜𝑙 𝐻2𝑂 = 1.11 𝑚𝑜𝑙 𝐻2𝑂 18.02 𝑔 𝐻2𝑂 g --> mol conversion 𝑔𝑟𝑎𝑚𝑠 𝑥 𝑚𝑜𝑙𝑒 𝑠𝑜𝑙𝑢𝑡𝑒 𝑙𝑖𝑡𝑒𝑟𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 Molarity 𝑀𝑜𝑙𝑎𝑟𝑖𝑡𝑦 = 20 𝑚𝑜𝑙𝑒𝑠 4.0 𝐿 When 20moles of solute is dissolved in 4.0 L of solution, a 5.0 M solution results 𝑀𝑎 𝑥 𝑉𝑎 = 𝑀𝑏 𝑥 𝑉𝑏 0.5𝑀 𝑥 100𝑚𝐿 = 0.25𝑀 𝑥 200𝑚𝐿 Titration The amount of moles in 100 mL of 5.0M solution is the same as that in twice the volume of diluted solution ½ the concentration Average 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝑜𝑓 𝐴&𝐵 = % 𝐸𝑟𝑟𝑜𝑟 = | Percent Error 5.0 = 𝐴+𝐵 2 𝑇ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙 − 𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙 | 𝑥100 𝑇ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙 5= 4% = | 4+6 2 25 − 24 | 𝑥100 25 The percent difference between the theoretical and experimental values were calculated as 4% Percent Acetic Acid in Vinegar 𝑚𝑜𝑙 𝑎𝑐𝑖𝑑 𝑔 𝑎𝑐𝑖𝑑 𝐿 𝐻2𝑂 𝑚𝐿 𝐻2𝑂 𝑥 𝑥 𝑥 𝑥100 𝐿 𝐻2𝑂 𝑚𝑜𝑙 𝑎𝑐𝑖𝑑 𝑚𝐿 𝐻2𝑂 𝑔𝐻2𝑂 𝑔 𝑎𝑐𝑖𝑑 = 𝑔 𝐻2𝑂 0.83 𝑚𝑜𝑙 𝑎𝑐𝑖𝑑 60.05 𝑎𝑐𝑖𝑑 𝐿 𝐻2𝑂 𝑥 𝑥 𝐿 𝐻2𝑂 𝑚𝑜𝑙 𝑎𝑐𝑖𝑑 1000𝑚𝐿 𝐻2𝑂 1 𝑚𝐿 𝐻2𝑂 𝑥 𝑥100 = 4.98% 1 𝑔𝐻2𝑂 The percent acetic acid in vinegar is found using the molarity of vinegar found during titration, the molar mass of vinegar, and known properties of water In order to solve for the percent acetic acid in vinegar, the units must be tracked and % AA by mass solved for







