IV. Electron Configuration
advertisement
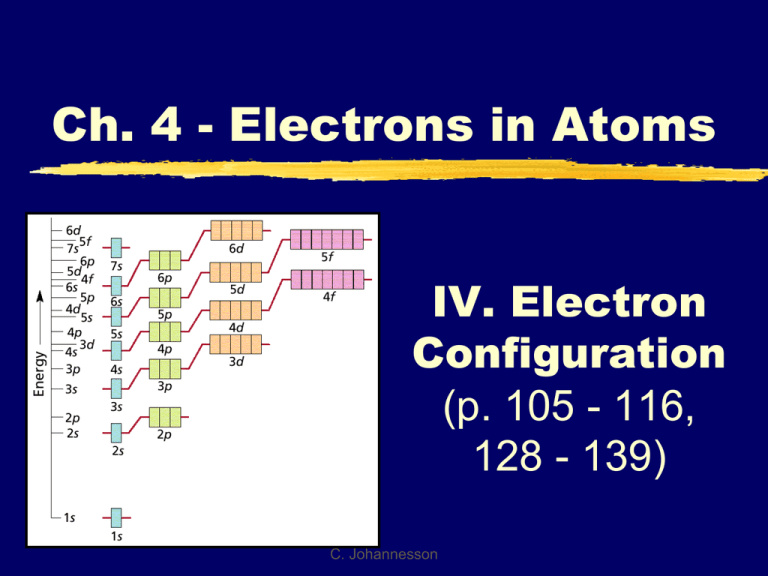
Ch. 4 - Electrons in Atoms IV. Electron Configuration (p. 105 - 116, 128 - 139) C. Johannesson Diagonal Rule The diagonal rule is a memory device that helps you remember the order of the filling of the orbitals from lowest energy to highest energy Aufbau principle states that electrons fill from the lowest possible energy to the highest energy Diagonal Rule 1 2 s s 4 s 5 s 7 Steps: 1. Write the energy levels top to bottom. 2. Write the orbitals in s, p, d, f order. Write 2p the same number of orbitals as the energy level. 3 s 6 3p 4p 3d 3. 4d 4. Draw diagonal lines from the top right to the bottom left. 4f To get the correct order, By this point, we are follow the arrows! past the current periodic 5p 5d 5f 5g? table so we can stop. s 6p 6d 6f 6g? 6h? s 7p 7d 7f 7g? 7h? 7i? Why are d and f orbitals always in lower energy levels? d and f orbitals require LARGE amounts of energy It’s better (lower in energy) to skip a sublevel that requires a large amount of energy (d and f orbitals) for one in a higher level but lower energy This is the reason for the diagonal rule! BE SURE TO FOLLOW THE ARROWS IN ORDER! C. Johannesson A. General Rules Pauli Exclusion Principle Each orbital can hold TWO electrons with opposite spins. C. Johannesson A. General Rules Aufbau Principle Electrons fill the lowest energy orbitals first. “Lazy Tenant Rule” C. Johannesson A. General Rules Hund’s Rule Within a sublevel, place one e- per orbital before pairing them. “Empty Bus Seat Rule” WRONG C. Johannesson RIGHT B. Notation Orbital Diagram O 8e- 1s 2s Electron Configuration 2 2 4 1s 2s 2p C. Johannesson 2p Draw these orbital diagrams! Carbon(C) Nitrogen (N) B. Notation Longhand Configuration S 16e- 1s2 2s2 2p6 3s2 3p4 Core Electrons Valence Electrons Shorthand Configuration S 16e 2 4 [Ne] 3s 3p C. Johannesson C. Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company C. Johannesson C. Periodic Patterns Period # energy level (subtract for d & f) A/B Group # total # of valence eColumn within sublevel block # of e- in sublevel C. Johannesson C. Periodic Patterns Example - Hydrogen 1 2 3 4 5 6 7 1st column of s-block 1 1s 1st Period s-block C. Johannesson Let’s Try It! Write the electron configuration for the following elements: H Li N Ne K Zn Pb Let’s Try It! Write the electron configuration for the following elements: H 1s1 Li 1s2 2s1 N 1s2 2s2 2p3 Ne 1s2 2s2 2p6 K 1s2 2s2 2p6 3s2 3p6 4s1 Zn1s2 2s2 2p6 3s2 3p6 4s2 3d10 Pb1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2 C. Periodic Patterns Shorthand Configuration Core e-: Go up one row and over to the Noble Gas. Valence e-: On the next row, fill in the # of e- in each sublevel. 1 2 3 4 5 6 7 C. Johannesson C. Periodic Patterns Example - Germanium 1 2 3 4 5 6 7 [Ar] 2 4s 10 3d C. Johannesson 2 4p D. Stability Full energy level Full sublevel (s, p, d, f) Half-full sublevel 1 2 3 4 5 6 7 C. Johannesson D. Stability Electron Configuration Exceptions Copper EXPECT: [Ar] 4s2 3d9 ACTUALLY: [Ar] 4s1 3d10 Copper gains stability with a full d-sublevel. C. Johannesson D. Stability Electron Configuration Exceptions Chromium EXPECT: [Ar] 4s2 3d4 ACTUALLY: [Ar] 4s1 3d5 Chromium gains stability with a half-full d-sublevel. C. Johannesson C. Johannesson Try These! Write the shorthand notation for: Cu W [Ar] 4s1 3d10 Au [Xe] 6s1 4f14 5d5 [Xe] 6s1 4f14 5d10 D. Stability Ion Formation Atoms gain or lose electrons to become more stable. Isoelectronic with the Noble Gases. 1 2 3 4 5 6 7 C. Johannesson D. Stability Ion Electron Configuration Write the e- config for the closest Noble Gas EX: Oxygen ion O2- Ne 2O 10e [He] C. Johannesson 2 2s 6 2p Keep an Eye On Those Ions! Electrons are lost or gained like they always are with ions… negative ions have gained electrons, positive ions have lost electrons The electrons that are lost or gained should be added/removed from the outermost energy level (not the highest orbital in energy!) Keep an Eye On Those Ions! Tin Atom: [Kr] 5s2 4d10 5p2 Sn+4 ion: [Kr] 4d10 Sn+2 ion: [Kr] 5s2 4d10 Note that the electrons came out of the outermost energy level, not the highest energy orbital! Keep an Eye On Those Ions! Bromine Atom: [Ar] 4s2 3d10 4p5 Br- ion: [Ar] 4s2 3d10 4p6 Note that the electrons went into the outermost energy level, not the highest energy orbital! Try Some Ions! Write the longhand notation for these: 2 2s2 2p6 1s F 2 + 1s Li 2 2s2 2p6 note this is the +2 1s Mg same as F- this is called isoelectronic Write the shorthand notation for these: Br[Ar]4s2 3d10 4p6 +2 Ba [Kr]5s2 4d10 5p6 Al+3 [He]2s2 2p6