Malignant transformation
advertisement

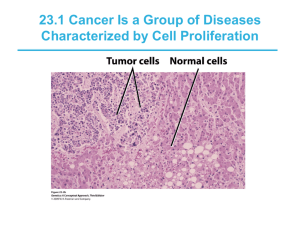
Malignant transformation October 11, 2011 Concepts of cell growth Growth of the body Renewal and repair Wound healing Cancer development Concepts of cell growth Proliferation Differentiation Apoptosis Neoplasia menas „new formation“ Tumor means swelling Proliferation Permanent cells Stabile cells Labile cells Cell differentiation Increase of specialization decrease of ability to undergo mitosis Stem cells – unspecialized cells surviving throughout life Progenitor cells - partially specialized Cell cycle Interval between each cell division G1,S,G2,M phases Checkpoints – fidelity of DNA dupliaction Benign and Malignant Neoplasms Malignant neoplasma: low level of differentiation, invasive growth, high rate of growth, metastases Tumor parenchyma x tumor stroma angiogenesis Tumor neoangiogenesis Dysequilibrium between antiangiogenetic and proangiogenetic factors („angiogenetic switch“). Without neoangigenetic switch, the tumor growth is possible only to 1 – 2 mm3, when O2 and nutrients support is possible by difussion from surrounding tissue. Hypoxia of tumor cells HIF- alpha induced transcription angiogenetic factors Invasiveness and Metastases Invasion of the surrounding tissues, lack of sharp demarcation. Metastases through lymphatic vessels, blood stream, dissemination in body cavities Neoplasm growth Exponential rate of growth Growth fraction Duration of cell cycle Cell death Doubling time Gompertz function Etiology of cancer Monoclonal theory of tumor origin Mutations in proto-oncogenes or tumor suppressor genes (apoptosis regulation) Genes coding DNA repair systems – indirect effect on oncogenesis Familial (inherited cancer) versus sporadic cancer Oncogenes Derived from proto-oncogenes by mutations Proto-oncogenes are normal genes needed for cell proliferation during growth, repair, replenishment of shed cells Growth factors genes GF receptors genes Protein kinases genes G proteins genes Transcription factors genes Oncogenes Mutations in proto-oncogenes are dominant i.e. one copy of the oncogene (mutated protooncogene) is sufficient to provoke tumor cell transformation Tumor suppressor genes Inhibit cell proliferation Suppressor genes are recessive i.e. one normal copy is sufficient to block tumor cell transformation Inherited cancers e.g. RB gene Tumor parenchyma x tumor stroma Tumor parenchyma – transformed cells with mutated DNA Tumor stroma – cells with normal genetic information Factors of carcinogenesis Heredity RB, BRCA-1, BRCA-2 Hormones Immunologic mechanisms Genital tract tumors Immune surveilllance hypothesis Tumors in immunocompromised patients (e.g. AIDS and Kaposi sarcoma) Chemical carcinogens 1775, scrotal cancer in chimney sweeps Industrial exposure Food and drugs Ionizing radiation Stochastic effects Oncogenic viruses Only a few of viruses is directly linked with cancer in humans HPV, EBV, HBV, HTLV-1 Monoclonal origin of the tumor Most human neoplasms are monoclonal in origin, i.e. they arise from genetic mutations within a single affected cell; however, over subsequent divisions heterogeneity develops through the accumulation of further abnormalities. The genes most commonly affected can be characterized as those controlling cell cycle check points, DNA repair and DNA damage recognition, apoptosis, differentiation, and growth signaling. Proliferation may continue at the expense of differentiation, which together with the failure of apoptosis leads to tumor formation with the accumulation of abnormal cells varying in size, shape and nuclear morphology as viewed down the light microscope. Cancer genetics The development of cancer is associated with a fundamental genetic change within the cell. Evidence for the genetic origin of cancer is based on the following: Some cancers show a familial predisposition. Most known carcinogens act through induced mutations. Susceptibility to some carcinogens depends on the ability of cellular enzymes to convert them to a mutagenic form. Genetically determined traits associated with a deficiency in the enzymes required for DNA repair are associated with an increased risk of cancer. Some cancers are associated with chromosome 'instability' because of deficiencies in mismatch repair genes. Many malignant tumors represent clonal proliferations of neoplastic cells. Many tumors contain well-described cytogenetic abnormalities, which involve mutated or abnormally regulated oncogenes and tumor suppressor genes with transforming activity in cell lines. Mutations may occur in the germ line and therefore be present in every cell in the body, or they may occur by somatic mutation in response, for example, to carcinogens, and therefore be present only in the cells of the tumor. Cancer genetics Expression of the mutation and hence carcinogenesis will depend upon the penetrance (due to level of expression and presence of other genetic events) of the gene and whether the mutated allele has a dominant or recessive effect. There are a small group of autosomal dominant inherited mutations such as RB (in retinoblastoma) and a small group of recessive mutations. Carriers of the recessive mutations are at risk of developing cancer if the second allele becomes mutated, leading to 'loss of heterozygosity' within the tumour although this is seldom sufficient as carcinogenesis is a multistep process. Cancer genetics Malignant transformation may result from a gain in function as cellular proto-oncogenes become mutated, (e.g. ras), amplified (e.g. HER2), or translocated (e.g. BCR-ABL). However, these mutations are insufficient to cause malignant transformation by themselves. Alternatively, there may be a loss of function of tumour suppressor genes that normally suppress growth and differentiation. A third mechanism involves alterations in the genes controlling the transcription of the oncogenes or tumour suppressor genes (e.g. Malignant transformation of the cells Cell division is carefully controled to serve to actual needs of the organism. Early in life, cell division capacity outweighs their destruction, in adulthood, it is in dynamic equilibrium and in senescence, involution dominates. Some cells are able to obviate the replication control.They change their phenotype to tumor ones. Benign tumor cells Malignant tumor cells (invasivity, metastatic potential). Malignant transformation of the cell Malignant tumor is a genetic disease It starts in one cell Usually a multistep process with Increasing number of alterations of genes controlling proliferation, diferenciation and destruction of the cells. Clonal progressing of tumor cells 1. mutation 2. Cells with mutation 1 and 2 gradually overgrow and/or displace cells with only mutation 1. 3. Next mutations cause the growth of more and more aggressive populations of the cells. 4. Subclonal genetic heterogenity of the tumor is a recoil of the progressing development of the tumor Malignant transformed cells Continue their division. Needs for presence of hormones and growth factors decreased. Production of own growth factors (autokrinnal stimulation). Decrement of ability to stop the growth. Decreased ability to stop the growth in worse nutrition conditions- Malignant transformed cells Occur most frequently in the tissues with quick proliferation, especially when the influence of kancerogenes and hormones is present. Environmental factors have a great impact on gene expression ot the target cells A plenty of signals accepted by the cell leads to activation of specific transcription factors which decide about either division or difecenciation or apoptosis Process of cancerogenesis Tři stádia: (1) Initial stadium, =mutation of critical gene. (2) Promotion stadium (years); Transformed cells are stimullated to even more intensive proliferation. Promotion factors are not able to initiate malignant transformation, only to give support to it. Process of cancerogenesis (3) Progression stadium - increased number of gene changes leading to (a) uncontroled growth due to permanent activation off signal transduction of growth stimulus, (b) alteration of check points of cell cycle (c) deregulation of DNA- transcription factors. Invasion Matastasis Tumor neoangiogenesis. Tumor phenotype Is characterised by the process of malignant transformation of the cell with - loss of control of cell division (alterations in cell cycle, antiapoptotic state, „immortality „ of tumor cell, alteration in signal transduction), - loss of cell-cell contant inhibition, invasivity, - changes in metabolism - proangiogenetic activation Malignant transformed cells seem to be resistent to external stress factors as hypoxia, low pH, hypoglycemia, malnutrition. Metastatic phenotype Loss of dependence to adhere to substrates (tumor cells are able to divide even in tissue culture) Loss of gap junctions. Changes in cell membrane (glycolipids and glycoproteins modifications). Metastatic phenotype Increased motility, invasion and ability to form metastases. Increased production of receptors (adhesive molecules) Increased production of hydrolytic enzymes Function of chemokines Tumorigenesis Phenomenon of tumor suport Chemical cancerogenes: Initiators: compounds with cancerogenic potential after their metabolisation (P450). Promoters: effects possible after initiators (phorbolester). Tumor-supresor protein p53 (TP53) Is a nuclear protein with key role between G0 and G1 phase of the cell cycle. Mutant variants of the p53 gene can be found in many cancer types. Somatic mutations Germ mutations (syndrome Li-Fraumeni). Tumor genotype Mutations in proto-onkogens tumor-supressor genes Genes for genome stability Genes modifiers. Proto-onkogenes and oncogenes Up to 100 of various human protooncogenes in each somatic cell. Targets for transduction signals (mitogenic), by which their expression is regulated Proto-onkogenes and oncogenes Mutations of proto-oncogenes changes them to oncogenes. Dominant mutation. Different pathways of proto-oncogenes activation: (a) viral transduction (e.g. onkogene src) (b) gene amplification (myc, abl), (c) viral insertion (myc) (d) chromosomal alteration (myc, abl) (e) mutation (ras) Types of oncogenes They are classified to 5 classes: (1) growth factors (2) growths factors receptors (3) intracellular transducers of the signals (4) nuclear transcription factors (5) proteins controlling cell cycle Apoptosis and tumorigenesis Overexpression of Bcl2 (B-cell lymphoma 2) gene was found to be related to inhibition of apoptosis during tumorigenesis. Tumors and immortality of cells Increased activity of telomerase. Telomers are key stabilisation factors of terminal part of the chromosome. Telomeres have repetitive sequence TAGGG. The length of telomers is decreased after multiple divisions of the cell (1 cell cycle is shortening of 1 telomere). Their renewal is catalysed by telomerase. Two mechanisms during tumorigenesis: mechanismus TERT („telomerase reverse transcriptase“) „alternative lengthening of telomere (ALT) pathway“),