LP AP Bio 8-23-10
advertisement
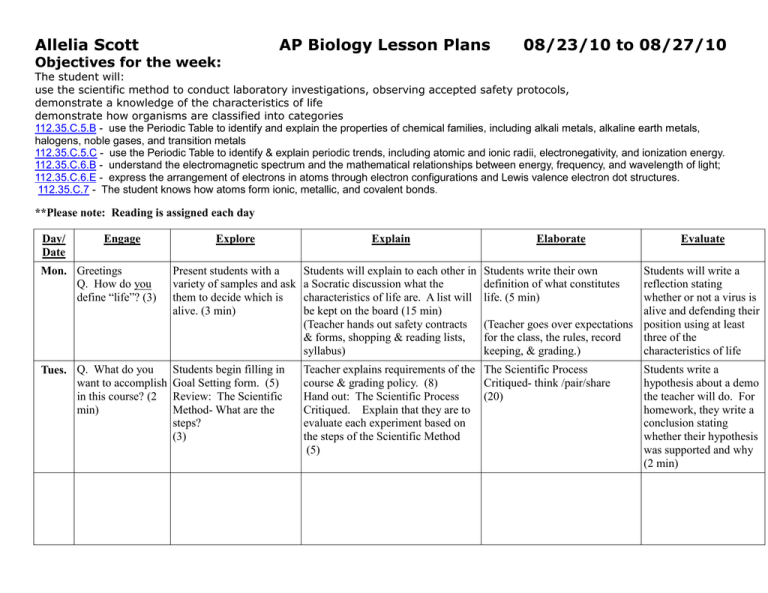
Allelia Scott Objectives for the week: AP Biology Lesson Plans 08/23/10 to 08/27/10 The student will: use the scientific method to conduct laboratory investigations, observing accepted safety protocols, demonstrate a knowledge of the characteristics of life demonstrate how organisms are classified into categories 112.35.C.5.B - use the Periodic Table to identify and explain the properties of chemical families, including alkali metals, alkaline earth metals, halogens, noble gases, and transition metals 112.35.C.5.C - use the Periodic Table to identify & explain periodic trends, including atomic and ionic radii, electronegativity, and ionization energy. 112.35.C.6.B - understand the electromagnetic spectrum and the mathematical relationships between energy, frequency, and wavelength of light; 112.35.C.6.E - express the arrangement of electrons in atoms through electron configurations and Lewis valence electron dot structures. 112.35.C.7 - The student knows how atoms form ionic, metallic, and covalent bonds. **Please note: Reading is assigned each day Day/ Date Engage Explore Explain Elaborate Mon. Greetings Q. How do you define “life”? (3) Present students with a variety of samples and ask them to decide which is alive. (3 min) Students will explain to each other in a Socratic discussion what the characteristics of life are. A list will be kept on the board (15 min) (Teacher hands out safety contracts & forms, shopping & reading lists, syllabus) Tues. Q. What do you want to accomplish in this course? (2 min) Students begin filling in Goal Setting form. (5) Review: The Scientific Method- What are the steps? (3) Teacher explains requirements of the The Scientific Process course & grading policy. (8) Critiqued- think /pair/share Hand out: The Scientific Process (20) Critiqued. Explain that they are to evaluate each experiment based on the steps of the Scientific Method (5) Evaluate Students write their own definition of what constitutes life. (5 min) Students will write a reflection stating whether or not a virus is alive and defending their (Teacher goes over expectations position using at least for the class, the rules, record three of the keeping, & grading.) characteristics of life Students write a hypothesis about a demo the teacher will do. For homework, they write a conclusion stating whether their hypothesis was supported and why (2 min) What do the terms Wed. What are the elements of a good (describe, compare, AP essay? (3 min) define, etc.) mean? Briefly go over handout on writing (5 min) Go over grading system for AP exams and show them the rubric for an example question. (5 min) HW Using the rubric as a check list, write an answer to the question. (question # 4 form 2004B AP exam) Students will use an activity kit to build models Using the periodic table, students of elements, ions, & will collaborate to make Lewis dot isotopes. (5) models of various elements. (10) Do “Comparing Molecular Thurs. Begin by taking quiz over Ch 1 & 2 Bonds” activity Q Which compounds have stronger chemical bonds- ionic or covalent? Why? Fri. Lab Activity: Physical properties of ionic, covalent & hydrogen bonds Teacher will brief the lab, then demonstrate the difference between melting points in ionic and covalent compounds and the conductivity of two solutions. Discussion: What trends are seen in the Periodic tableconcepts- electronegativity, the number of electrons that fill each shell, valence electrons, families & periods, bond types Announce quiz for tomorrow over Ch 1 & 2 Drawings of atoms and atomic bonding will be on the quiz. HW Using Lewis dot diagrams, show how covalent and ionic compounds form. Answer questions Lab Activity: Ionic vs. Covalent compoundscomparison of the physical and chemical properties of ionic & covalent compounds HW Do free response essay on radioisotopes Watch assigned videos Get chapter notes caught up (Notebook check on Monday) Lab Activity: (continued) Ionic vs. Covalent compoundscomparison of the physical and chemical properties of ionic & covalent compounds Answer reflection questions on Molecular Bonds. Write a summary on what was discovered in this activity. Reflection: Students will answer reflection questions and write a summary of what they have learned about ionic and covalent compounds HW Begin Tic Tac Toe Activity in terms of relative strength, conductivity, melting point, etc..




