2003 AP CHEM FREE RESPONSE QUESTION #2
advertisement

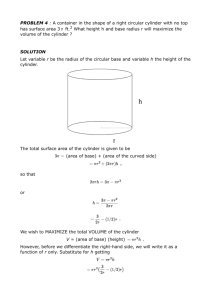
AP CHEM FREE RESPONSE Q #5 1999 Your response to the question in this part of the examination will be graded on the basis of the accuracy and relevance of the information cited. Explanations should be clear and well organized. Examples and equations may be included in your responses where appropriate. Specific answers are preferable to broad, diffuse responses. 5. A student performs an experiment to determine the molar mass of an unknown gas. A small amount of the pure gas is released from a pressured container and collected in a graduated tube over water at room temperature, as shown in the diagram above. The collection tube containing the gas is allowed to stand for several minutes, and its depth is adjusted until the water levels inside and outside the tube are the same. Assume that: • • the gas is not appreciably soluble in water the gas collected in the graduated tube and the water are in thermal equilibrium • a barometer, a thermometer, and analytical balance, and a table of the equilibrium vapor pressure of water at various temperatures are also available. (a) (b) (c) (d) Write the equation(s) needed to calculate the molar mass of the gas. List the measurements that must be made in order to calculate the molar mass of the gas. Explain the purpose of equalizing the water levels inside and outside the gas collection tube. The student determines the molar mass of the gas to be 64 g mol–1. Write the expression (setup) for calculating the percent error in the experimental value, assuming that the unknown gas is butane (molar mass 58 g mol–1). Calculations are not required. (e) If the student fails to use information from the table of the equilibrium vapor pressures of water in the calculation, the calculated value for the molar mass of the unknown gas will be smaller than the actual value. Explain. 2005 AP CHEMISTRY FREE-RESPONSE QUESTION #2 Answer EITHER Question 2 below OR Question 3 printed on page 8-9. Only one of these two questions will be graded. If you start both questions, be sure to cross out the question you do not want graded. The Section II score weighting for the question you choose is 20 percent. 2. Answer the following questions about a pure compound that contains only carbon, hydrogen, and oxygen. (a) A 0.7549 g sample of the compound burns in O2(g) to produce 1.9061 g of CO2(g) and 0.3370 g of H20(g). (i) Calculate the individual masses of C, H, and O in the 0.7549 g sample. (ii) Determine the empirical formula for the compound. (b) A 0.5246 g sample of the compound was dissolved in 10.0012 g of lauric acid, and it was determined that the freezing point of the lauric acid was lowered by 1.68°C. The value of Kf of lauric acid is 3.90°C m-l. Assume that the compound does not dissociate in lauric acid. (i) Calculate the molality of the compound dissolved in the lauric acid. (ii) Calculate the molar mass of the compound from the information provided. (c) Without doing any calculations, explain how to determine the molecular formula of the compound based on the answers to parts (a)(ii) and (b)(ii). (d) Further tests indicate that a 0.10 M aqueous solution of the compound has a pH of 2.6. Identify the organic functional group that accounts for this pH. AP CHEMISTRY 2006 Question 3 3. Answer the following questions that relate to the analysis of chemical compounds. (a) A compound containing the elements C, H, N, and 0 is analyzed. When a 1.2359 g sample is burned in excess oxygen, 2.241 g of CO2(g) is formed. The combustion analysis also showed that the sample contained 0.0648 g of H. (i) Determine the mass, in grams, of C in the 1.2359 g sample of the compound. (ii) When the compound is analyzed for N content only, the mass percent of N is found to be 28.84 percent. Determine the mass, in grams, of N in the original 1.2359 g sample of the compound. (iii) Determine the mass, in grams, of 0 in the original 1.2359 g sample of the compound. (iv) Determine the empirical formula of the compound. (b) A different compound, which has the empirical formula CH2Br, has a vapor density of 6.00 g L-I at 375 K and 0.983 atm. Using these data, determine the following. (i) The molar mass of the compound (ii) The molecular formula of the compound 2006 The College Board 2003 AP CHEM FREE RESPONSE QUESTION #2 (Assignment 1) Answer EITHER Question 2 below or Question 3 printed on page 8. Only one of these two questions will be graded. If you start both questions, be sure to cross out the question you do not want graded. The Section II score weighting for the question you choose is 20 percent. 2. A rigid 5.00 L cylinder contains 24.5 g of N2(g) and 28.0 g of 02(g). (a) Calculate the total pressure, in atm, of the gas mixture in the cylinder at 298 K. (b) The temperature of the gas mixture in the cylinder is decreased to 280 K. Calculate each of the following. (i) The mole fraction of N2(g) in the cylinder (ii) The partial pressure, in atm, of N2(g) in the cylinder (c) If the cylinder develops a pinhole-sized leak and some of the gaseous mixture escapes, would the ratio moles of N2(g) in the cylinder increase, decrease, or remain the same? moIes of O 2(g) Justify your answer. A different rigid 5.00 L cylinder contains 0.176 mol of NO(g) at 298 K. A 0.176 mol sample of 02(g) is added to the cylinder, where a reaction occurs to produce NO2(g). (d) Write the balanced equation for the reaction. (e) Calculate the total pressure, in atm, in the cylinder at 298 K after the reaction is complete. 2000 AP Chemistry Free Response Question # 3 Assignment 1 3. Answer the following questions about BeC2O4(s) and its hydrate. (a) Calculate the mass percent of carbon in the hydrated form of the solid that has the formula BeC2O4 . 3 H2O (b) When heated to 220.oC BeC2O4 . 3 H2O(s) dehydrates completely as represented below. BeC2O4 . 3 H2O(s) → BeC2O4(s) + 3 H2O (g) If 3.21 g of BeC2O4 . 3 H2O(s) is heated to 220.oC, calculate (i) the mass of BeC2O4(s) formed, and, (ii) the volume of the H2O(g) released, measured at 220.oC and 735 mm Hg. (c) A 0.345 g sample of anhydrous BeC2O4, which contains an inert impurity, was dissolved in sufficient water to produce 100. mL of solution. A 20.0 mL portion of the solution was titrated with KMn04(aq). The balanced equation for the reaction that occurred is as follows. 16H+(aq) + 2 Mn041-(aq) +5 C2O42- (aq) → 2 Mn2+(aq) + 10 CO2(g) + 8 H20(l). The volume of 0.0150 M KMn04(aq) required to reach the equivalence point was 17.80 mL. (i) Identify the reducing agent in the titration reaction. (ii) For the titration at the equivalence point, calculate the number of moles of each of the following that reacted. Mn041-(aq) C2O42- (aq) (iii) Calculate the total number of moles of C2O42- (aq) that were present in the 100. mL of prepared solution. (iv) Calculate the mass percent of BeC204(s) in the impure 0.345 g sample. 2003 AP CHEM FREE RESPONSE QUESTION #2 (Assignment 1) Answer EITHER Question 2 below or Question 3 printed on page 8. Only one of these two questions will be graded. If you start both questions, be sure to cross out the question you do not want graded. The Section II score weighting for the question you choose is 20 percent. 2. A rigid 5.00 L cylinder contains 24.5 g of N2(g) and 28.0 g of 02(g). (a) Calculate the total pressure, in atm, of the gas mixture in the cylinder at 298 K. (b) The temperature of the gas mixture in the cylinder is decreased to 280 K. Calculate each of the following. (i) The mole fraction of N2(g) in the cylinder (ii) The partial pressure, in atm, of N2(g) in the cylinder (c) If the cylinder develops a pinhole-sized leak and some of the gaseous mixture escapes, would the ratio moles of N2(g) in the cylinder increase, decrease, or remain the same? moIes of O 2(g) Justify your answer. A different rigid 5.00 L cylinder contains 0.176 mol of NO(g) at 298 K. A 0.176 mol sample of 02(g) is added to the cylinder, where a reaction occurs to produce NO2(g). (d) Write the balanced equation for the reaction. (e) Calculate the total pressure, in atm, in the cylinder at 298 K after the reaction is complete.