DENSITY ppt
advertisement

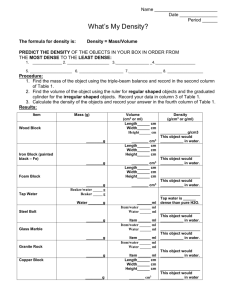
On page 17 in your Notebook write the two Essential Questions (EQ) below DENSITY How does an object float? How is density determined? RTI 1. What does it mean to be organized? 2. How can being organized help a person to be successful? 3. Are you organized? If so how? If not, why? 4. What are 5 things you can do to assist you with being organized? RTI: RESPECT 1. What does respect mean? 2. What does it look like when you are in the classroom? 3. How do you personally show respect to others? (this is what you yourself do when you are being respectful) 4. How do you want others to show respect to you? Warm Up: What do you know? You will complete either the “floating logs” or the “Comparing Cubes” assessment probe. Be sure to write clear and complete sentences. You will present what you found to the class as a group. You will have 5 minutes to gather your thoughts. Let’s Get it Started • Regular coke versus Diet Coke • Research Question (what do we want to know): • Hypothesis (will answer the research question by telling what we will do and what we expect to happen) • Observation (what similarities and differences do you see with the diet coke and the regular coke), what do you observe happening when they are placed in the water? • Conclusion (was your hypothesis correct? Why or why not?) • Extension: what events can occur to cause the experiment to have mistakes or errors? Vocabulary (write on page 18) Density: the ratio of mass (how much matter it has in it) of an object to its volume (how much space the object takes up) Mass: amount of matter in an object. It is measured in grams Volume: amount of space occupied by mass. It is measured in liters. Grams: unit of measure used for mass. Metric symbol=(g) EX. 1g=1000mg (milligrams), Liter: unit of measure used to measure liquid or gas. Metric symbol is (L) 1L=1000mL (milliliters) Meter: unit of measure used to measure distance or length. Metric symbol (m) Ex. 1m=1000mm (millimeters) Density Essential Question (EQ): How is density determined? • Learning Objectives (after this lesson) • I will learn the molecular structure of the three states of matter: solid, liquid and gas • I will be able to understand that density will not change as the size and shape of an object changes as long as the material is the same material • If the mass of an object increases and the volume remains the same, the density of the object will increase. If the volume increases and the mass remains the same, the density of the object will decrease. • Water has a density of 1g/cm3 • I will be able to define and calculate the density of an object by using its formula. I will be able to explain how density affects whether an object will float or if it will sink. Oil and Water Activity Get the mass of empty beaker using the triple beam scale Get the mass of beaker with 30 ml of water Get the mass of oil, water and beaker Get the volume of the oil and water by looking at the beaker and reading the volume in ml Formula for finding the density of an object Mass ÷ volume or it can be written as mass volume Water Has a density of 1g/cm3 What does this mean? This means that if you have a cubic meter of water (an amount of water that is one meter in height, one meter in width, and one meter in depth), that amount of water would weigh 1000 kilograms or one gram. So any object with a density greater than 1g/cm3 is said to be more dense than So any object with a density greater than 1g/cm3 is said to be more dense than water and will sink Any object less than 1g/cm3 will float on top of water One way to look at density Matter can be found in different forms: SOLID, LIQUID or GAS. Each form has density based on the molecules that make up the object The more the molecules are packed together, the MORE DENSE the object will be. The more loosely packed the molecules are in an object, the LESS DENSE the object will be Ex. Snow When it is packed into a snowball it is more dense than if it is just lying loosely on the ground. How to calculate the density (formula m/v or m÷v) 1. Find the mass of an object by placing it on the scale 2. Find the volume of an object by using a beaker measuring in mL or a graduated cylinder measuring in mL. 3. Divide the mass of that object by its volume Calculating DENSITY….practice • Ex. The mass of an object is 25 grams. The volume of an object is 5 cm3. What is the density? • 25g÷5cm3= • The mass of an object is 40g. The volume of the object is 10ml. What is the density? How to find the volume of a cube • Measure the length of the cube, measure the height of the cube and measure the width of the cube. L x W x H • What is the volume of the cube to the left? Practice: a. What is the volume of a cube that has a length of 3, width of 3 and a height of 3. b. What is the volume of a cube which has a height of 6 and a width of 6 and a length of 6 How to find the volume of an irregular shape object • To find the volume of an object that does not have length, width or height we will use a system called WATER DISPLACEMENT. • This is the process in which a given amount of water is placed in a graduated cylinder and recorded. • An object is then placed within the graduated cylinder and the volume is recorded. • Subtract the volume of the water from the volume of the water and the object. The difference is the volume of the object. LET’S PRACTICE How do you find the mass of an object • Place the object on a triple beam scale and move the beams Summary • Shape up: Identify one thing you loved about today’s activities Write down four main ideas/concepts about density you learned today in class Write down 3 facts you learned today about density Write one sentence to summarize the main focus for today’s lesson on density.